Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 25
May 9th, 2018
CASE II: 16A351 (JPC 4101577).
Signalment: Full-term stillborn male rhesus macaque (Macaca mulatta), non-human primate.
History: A 4-year-old primiparous, outdoor-housed female rhesus macaque from a large breeding colony presented acutely for clinical signs of dystocia. Ultrasonography revealed an absent fetal heartbeat consistent with in utero fetal demise. A caesarean section was subsequently performed; the dam recovered uneventfully. A full-term stillborn male rhesus macaque was submitted for necropsy along with the bidiscoid placenta.
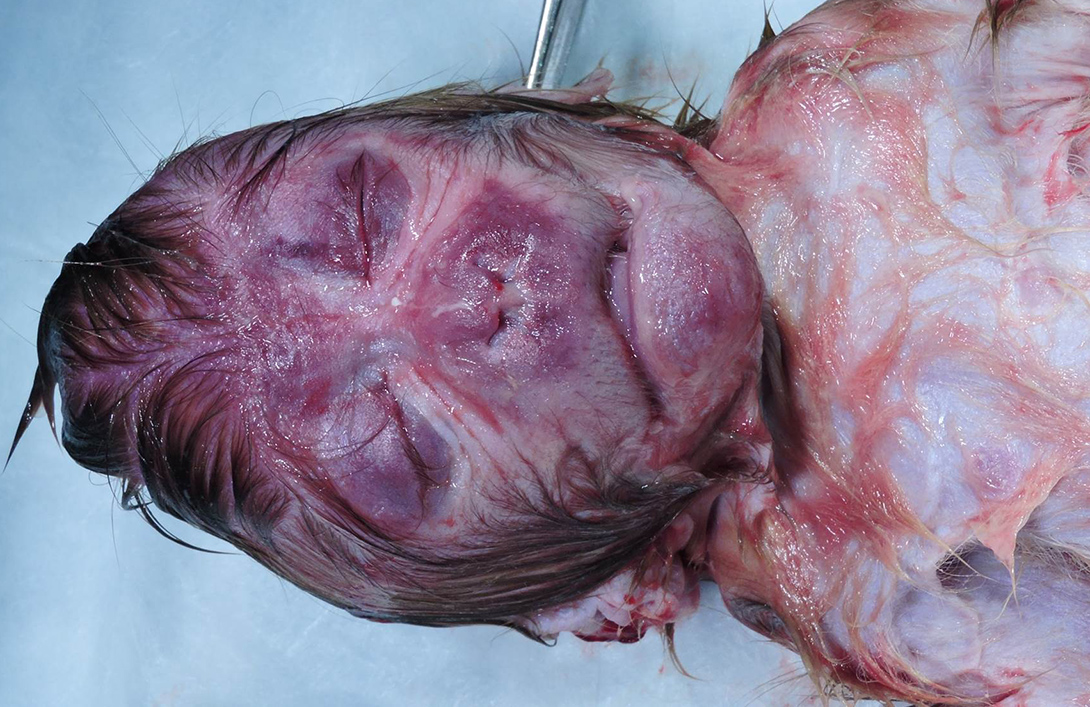
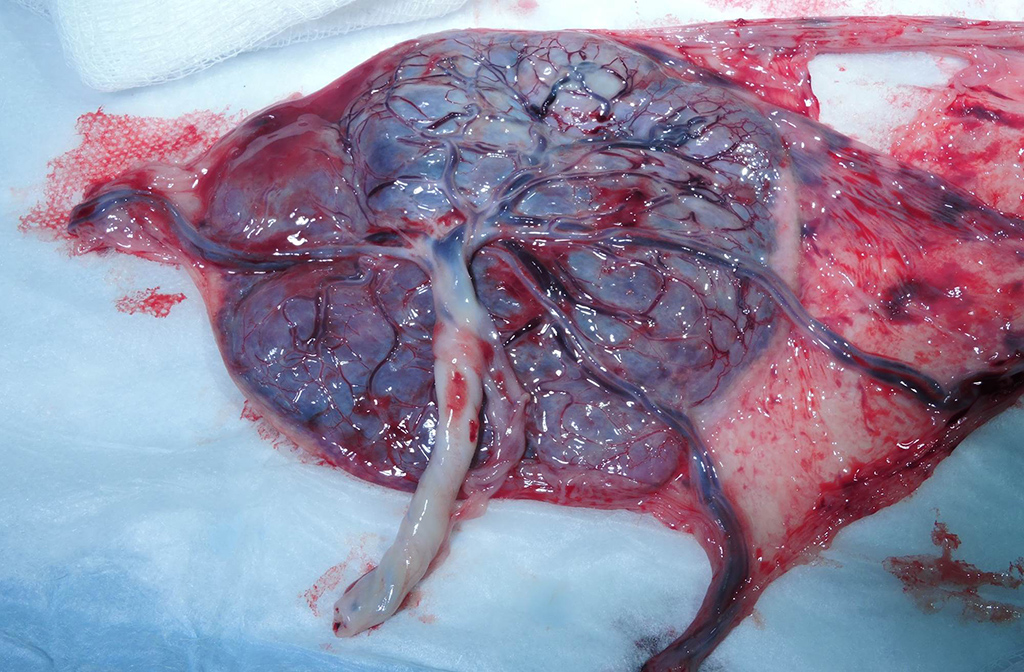
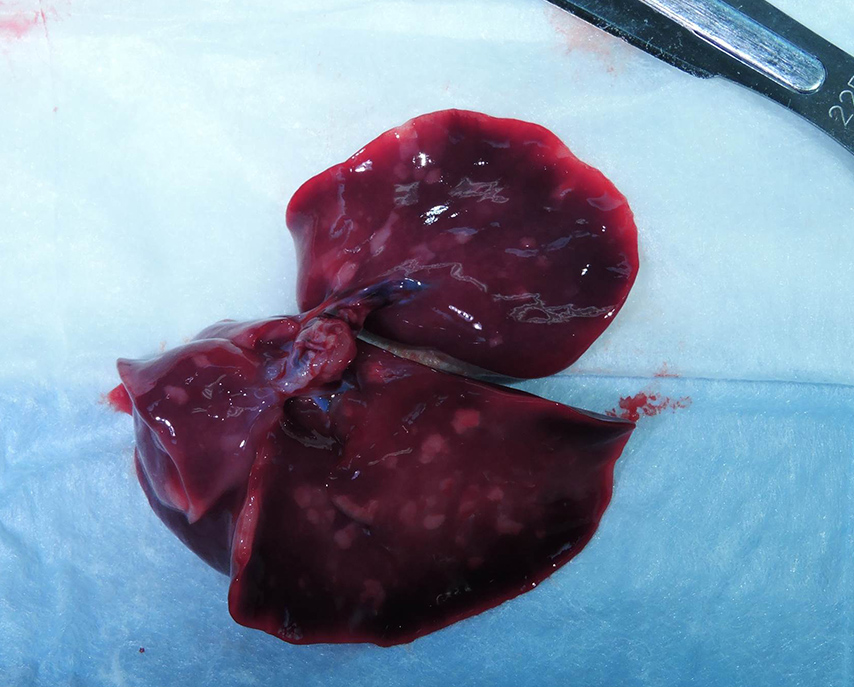
Gross Pathology: Grossly, the crown of the head of the fetus was deformed and exhibited abundant subcutaneous edema and hemorrhage. The face demonstrated moderate edema and congestion. The lungs were diffusely deep red and atelectatic, with scattered 1- 2mm white foci. Gross examination of the primary and secondary placental discs revealed few opaque tan-white foci on the fetal surface with slight thickening of the fetal membranes. Multifocally, there were marginal regions of pallor and infarction on both placental discs.
Laboratory results: 4+ beta-hemolytic Streptococcus sp. was isolated from the placenta and stomach, with 2+ beta-hemolytic Streptococcus sp. cultured from the pleura
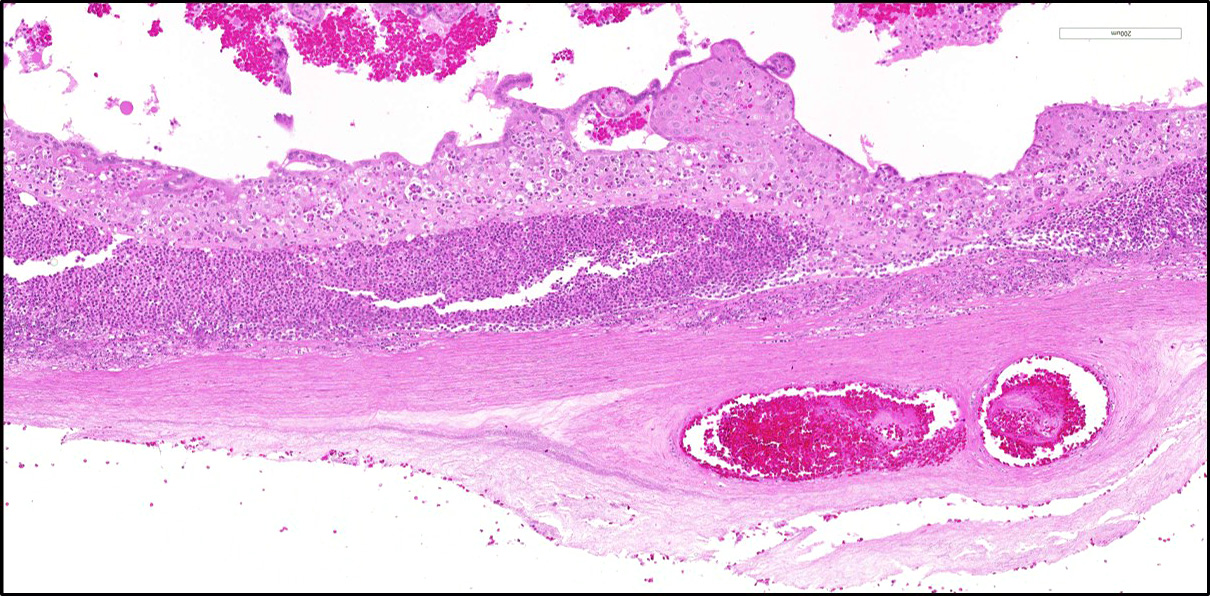
Microscopic Description: Two sections of placenta (primary disc) with attached fetal membranes are examined.
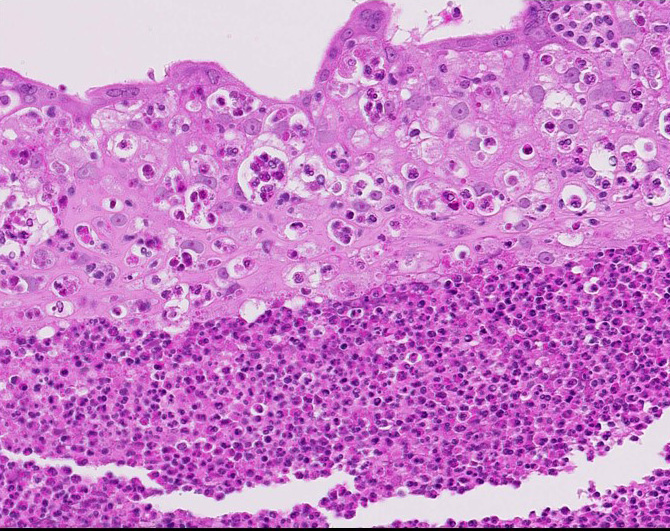
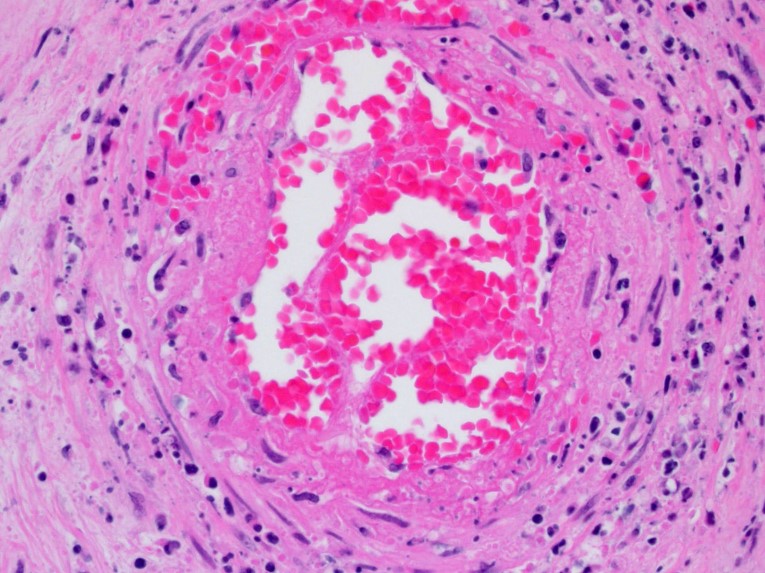
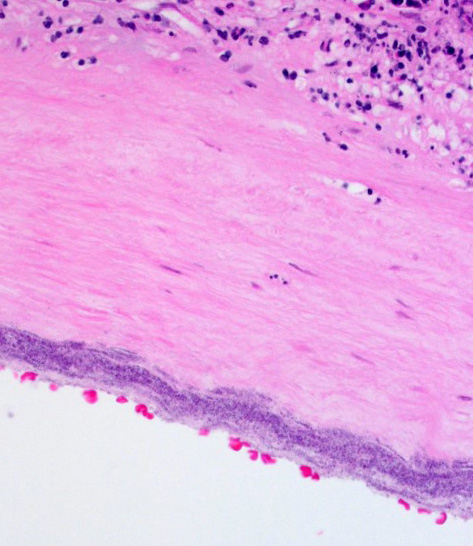
Diffusely expanding and infiltrating the subchorionic trophoblast layer is a nearly continuous, thick band of karyorrhectic neutrophils admixed with eosinophilic cellular debris, and few clusters of homogenous eosinophilic material (fibrin). Trophoblasts are vacuolated and necrotic. The chorionic plate is expanded by edema and contains fewer numbers of neutrophils which are often perivascular. The amniotic epithelium is sloughed and replaced by necrotic cellular debris interspersed with wispy strands of eosinophilic material. High numbers of cocci are found at the interface of the amnion and chorion, extending into the subjacent chorionic plate. Chorionic (fetal) blood vessels are infiltrated by karyorrhectic neutrophils and vessel walls are smudgy and hypereosinophilic (fibrinoid necrosis). Few large- and medium-size vessels contain luminal fibrin thrombi. The fetal membranes are diffusely necrotic and multifocally expanded by high numbers of karyorrhectic neutrophils and eosinophilic cellular debris. The maternal basal plate and decidua contain lower numbers of degenerate neutrophils which occasionally surround maternal blood vessels. Numerous fibrin coagula are also present within the basal plate. There is moderate, multifocally extensive extravasation of red blood cells (acute hemorrhage) along the maternal decidua on both tissue sections.
Contributor’s Morphologic Diagnosis: Placenta, fetal membranes: Chorioamnionitis, neutrophilic, necrotizing, acute, diffuse, severe, with subchorionic microabscesses, chorionic necrotizing vasculitis, amniotic epithelial necrosis, mild neutrophilic deciduitis, decidual vasculitis, and gram-positive cocci, rhesus macaque (Macaca mulatta), nonhuman primate.
Other morphologic diagnoses:
- Umbilical cord: Funisitis, neutrophilic, necrotizing, marked, multifocal with segmental necrotizing umbilical arteritis.
- Lung: Bronchopneumonia, pyogranulomatous, subacute, moderate, diffuse with peribronchial hemorrhage, high numbers of intralesional cocci, intra-alveolar squamous cells, mucin, and amorphous debris.
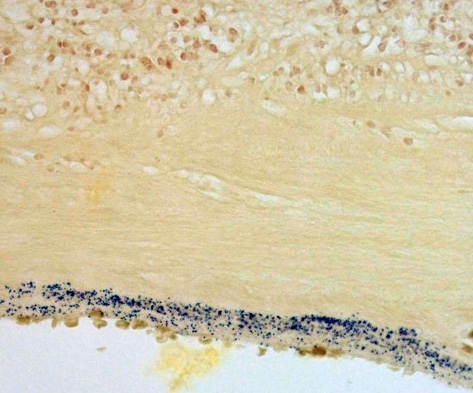
Contributor’s Comment: This is a case of acute streptococcal chorioamnionitis with fetal demise. Microbial culture of the placenta yielded 4+ beta-hemolytic Streptococcus sp. Gram staining of placenta sections confirmed numerous gram-positive cocci within the fetal membranes. The umbilical cord and umbilical vessels also contained neutrophilic infiltrates and exhibited necrotic changes to umbilical vessel walls. The character and distribution of inflammation within the fetal membranes in this case, is consistent with acute chorioamnionitis (ACA) due to ascending bacterial infection from the dam's lower genital tract.
In humans and non-human primates, ACA consists of a fetal and maternal acute inflammatory response characterized by diffuse neutrophilic infiltration variably involving the fetal membranes, placenta, and umbilical cord. The initial inflammatory response is maternal, with neutrophils exiting the placental intervillous space and decidual vessels with infiltration of the chorionic plate, later migrating through the fetal membranes and eventually into the amniotic fluid.1,2 As the maternal inflammatory response progresses, neutrophils become karyorrhectic and apoptotic. In contrast, the fetal inflammatory response varies depending on the gestational age, but may be manifested as chorionic vasculitis, umbilical arteritis and phlebitis, and funisitis as neutrophils transmigrate across the umbilical vessels.
Most acute chorioamnionitis cases in humans and non-human primates are considered to have an infectious etiology. Ascending microbial invasion from the lower genital tract is the most common route of intrauterine and subsequent intra-amniotic infection.1,5 Following infection of the amniotic cavity, the fetus may aspirate infected amniotic fluid leading to fetal pneumonia, sepsis, and death in-utero. Infectious pathogens within amniotic fluid may also be swallowed into the gastrointestinal tract or may gain access through the middle ear and tympanic membrane. Commonly isolated microorganisms in human placentas with a histologic diagnosis of ACA include vaginal commensals such as streptococci, staphylococci, Ureaplasma sp., and Mycoplasma hominis, as well enteric bacteria and anaerobes including Bacteroides sp., E.coli, and Fusobacterium sp..1,5,7 In addition to commensal organisms from the lower reproductive tract, Listeria monocytogenes, a sporadic cause of abortion and stillbirth in captive macaques, may also produce a neutrophilic and necrotizing placentitis characterized by intervillositis and villitis. L. monocytogenes undergoes hematogenous spread, gaining access to the placenta through the maternal circulation and intervillous space and eventually invading the villi and fetal circulation.
Acute chorioamnionitis also commonly and indirectly contributes to fetal morbidity and mortality by precipitating preterm labor and birth. Phospholipase production by invading bacteria and neutrophils stimulates the synthesis of prostaglandins, which induce uterine contractions and cervical dilation.6,7 Increased matrix metalloproteinases (MMP) may contribute to degradation of the extracellular matrix of the fetal membranes, potentially resulting in premature membrane rupture. Up to one-third of preterm deliveries in women are attributed to ACA.7
More frequently, acute chorioamnionitis remains subclinical in the mother with occasional clinical manifestations including pyrexia, leukocytosis, purulent amniotic fluid or vaginal discharge, and uterine tenderness. Maternal sepsis is infrequent and the primary clinical impacts of ACA are the adverse effects on the fetus.5
Histologic changes in the present case mirror many of the classic features of ACA found in human placentas. The degree of neutrophil karyorrhexis and amniotic epithelial necrosis coupled with necrotizing funisitis on sections of umbilicus (not included) are indicative of later-stage and more severe infection.1 Beta-hemolytic Streptococcus sp. was isolated from the stomach and pleura of this full-term stillborn rhesus macaque, consistent with ingestion and aspiration of contaminated amniotic fluid. Gram staining of lung sections confirmed high numbers of gram- positive cocci within the alveoli and small airways. Microscopic evaluation of fetal lung tissue showed a pyogranulomatous bronchopneumonia with increased numbers of squamous cells, mucin, and debris. Acute aspiration of meconium was also considered as an etiology for the bronchopneumonia, however, meconium pigment was not appreciated microscopically on lung sections.
Infections due to beta-hemolytic Streptococcus sp. in nonhuman primates, including the present case, are considered largely opportunistic and depend on host factors such as age, pregnancy and immune system status, as well as environment and social stress.3 The most pathogenic streptococcal groups in macaques are beta-hemolytic groups A and B as well as alpha-hemolytic Streptococcus pneumoniae, which lacks the Lancefield antigen and serogroup designation. In addition to reproductive loss, streptococcal infections in macaques have also been reported to cause suppurative pneumonia, rhinitis, conjunctivitis, meningoencephalitis, polyarthritis, sepsis, and polyserositis.
JPC Diagnosis: Placental disc and chorioamnionic membrane: Chorioamnionitis and deciduitis, neutrophilic and necrotizing, subacute, diffuse, severe, with necrotizing vasculitis, loss of amniotic epithelium and numerous cocci.
Conference Comment: The contributor has done an outstanding job in describing this case.
Streptococci are gram-positive, catalase-negative bacteria which are arranged in pairs or chains and exhibit varying degrees of hemolysis on blood agar which has been used to group the different species. α-Hemolytic streptococci produce a green zone around the colonies from hydrogen peroxide oxidation of hemoglobin to methemoglobin but do not lyse erythrocytes. β-Hemolytic streptococci tend to be the most pathogenic types and cause acute lysis of red blood cells with hemolytic zones around colonies on blood agar. Finally, γ-streptococci are non-hemolytic and non-pathogenic. Another method to divide the over 98 recognized species of Streptococcus is using a precipitin test based on extractable carbohydrate antigens called Lancefield groups which are designated A through V (excluding I and J). These groups are further subdivided based on protein antigens, M, R, and T. This is of some importance clinically, as antibodies to Lancefield groups are not protective but antibodies to M, R, or T are protective. M protein, in particular, is an important virulence determinant because it allows the bacterium to evade phagocytosis. For example, the SeM protein of Streptococcus equi ssp. equi prevents phagocytosis by binding host immunoglobulin and coating the bacterium, masking sites for complement activation and collectin/ficolin binding. Another protein, the FbsA protein is a fibrinogen-binding protein which aids in virulence of Streptococcus agalactiae. Other streptococci have analogous proteins, like the FOG protein in group G Streptococcus dysgalactiae ssp. equisimilis, and the Szp protein of S. equi ssp. zooepidemicus. Most species of virulent streptococci have a capsule which is either composed of hyaluronic acid (groups A and C) or polysaccharide (groups B, E, and G). The gram-positive cell wall of streptococci contain lipoteichoic acids and peptidoglycan which interact with host macrophages and induce release of proinflammatory cytokines.9
Particularly virulent streptococcal species possess pyrogenic toxin superantigens. Superantigens simultaneously bind with major histocompatibility complex class II molecules and T cell receptor molecules on the V-β region resulting in massive activation of antigen-presenting cells and T-cells which proceed to produce overwhelming amounts of cytokines. This so-called “cytokine storm” has detrimental systemic effects and often results in the death of the animal.9
Contributing Institution:Oregon Health & Science University
Oregon National Primate Research Center
Division of Comparative Medicine Pathology Services Unit
505 NW 185th Avenue Beaverton, OR 97006
www.ohsu.edu/xd/research/centers-institutes/onprc
References:
- Benirschke K, Burton GJ, Baergen RN. Infectious diseases of the placenta. In: Pathology of the Human Placenta. 6th ed. New York, NY: Springer; 2012:557- 581.
- Chong JK, Roberto R, Chaemsaithong P, et al. Acute chorioamnionitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015; 213(4 Suppl):529-552.
- Cline JM, Brignolo L, Ford EW. Urogenital System. In: Abee CR, Mansfield K, Tardif S, Morris T. eds. Nonhuman Primates in Biomedical Research: Diseases. 2nd ed. Vol 2. Waltham, MA: Elsevier; 2012:524-527.
- Egal AS, Ardeshir A, Mariano FV, et al. Contribution of endemic listeriosis to spontaneous abortion and stillbirth in a large outdoor-housed colony of rhesus macaques (Macaca mulatta). J Am Assoc Lab Animal Sci. 2015; 54(4):399-404.
- Gersell DJ, Kraus FT. Diseases of the placenta. In: Kurman RJ, Ellanson LH, Ronnett BM, eds. Blausteins's Pathology of the Female Genital Tract. 6th ed. New York, NY: Springer; 2011:1036-1041.
- Gravett MG, Witkin SS, Novy MJ. A non-human primate model for chorioamnionitis and preterm labor. Semin Reprod Endocrinol. 1994; 12(14):246-262.
- Novy MJ, Duffy L, Axthelm MK et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56-70.
- Saji F, Samejima Y, Kamiura S, et al. Cytokine production in chorioamnionitis. J Reprod lmmunol. 2000; 47(2):185-196.
- Steward GC. Streptococcus and Enterococcus. In: McVey DS, Kennedy M, Chengappa MM, eds. Veterinary Microbiology. 3rd Ames, IA: John Wiley & Sons, Inc.; 2013:194-199.