Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 19
19 February, 2020
Dr.
Molly Church, DVM, PhD, DACVP
Assistant Professor
Pathobiology
University of Pennsylvania School of Veterinary Medicine
Philadelphia, PA
CASE I: 17041318 (JPC 4117490).
Signalment: 3-month-old, female, Aberdeen-Angus calf (Bos taurus)
History: Paraparesis and inability to stand with the hindlimbs were noted for 6 days. Clinical signs were not improved with penicillin treatments and progressed to lateral recumbency and hindlimb paralysis. Rigidity of the head and neck was noted. The calf had no deep pain and no patellar reflex in both hindlimbs, whereas deep pain was normal in the forelimbs. Cranial nerve responses were intact and normal. Omphalitis was present, and body temperature, pulse and respiratory rates were normal. The calf was euthanized and submitted for necropsy.
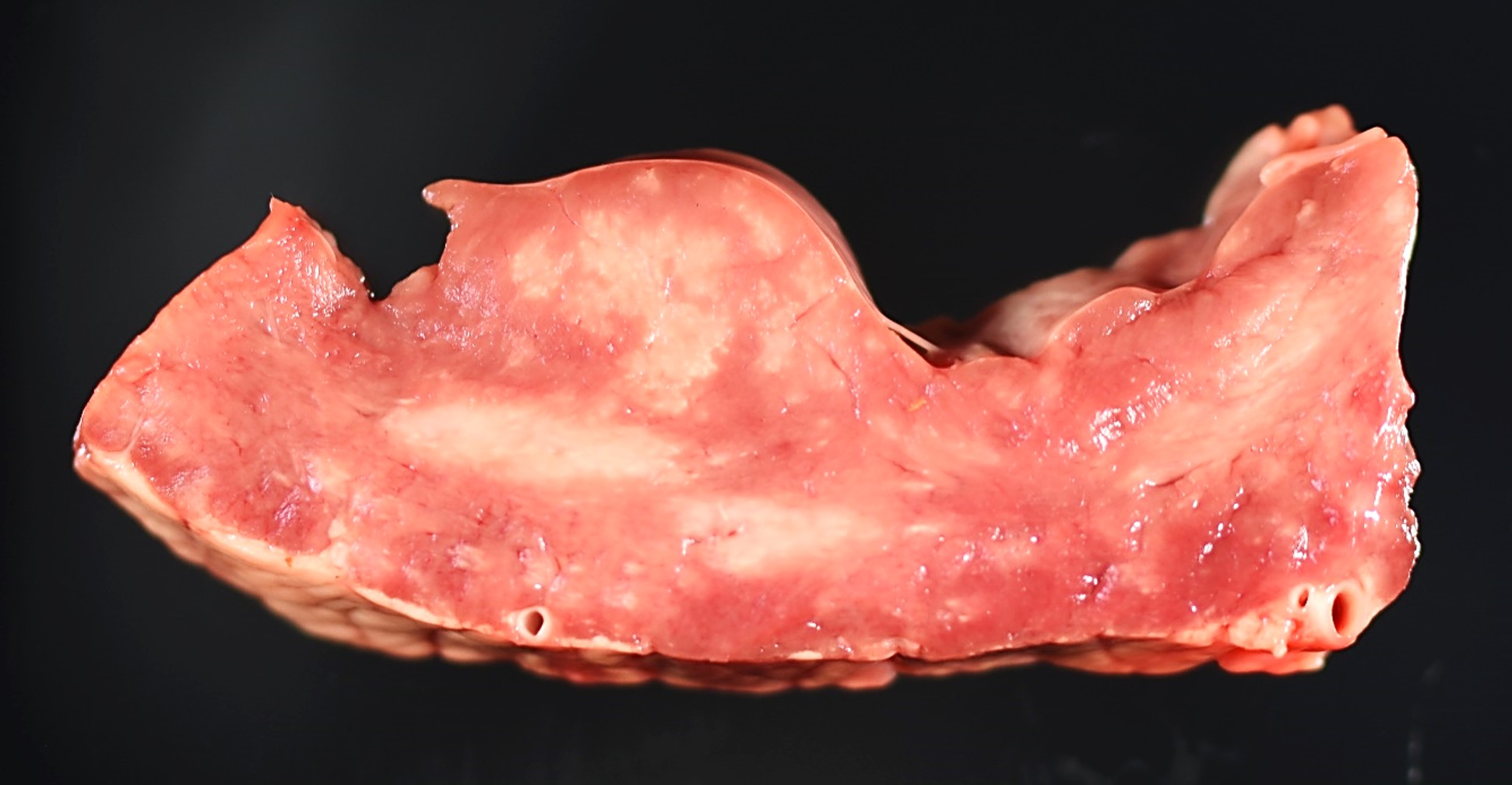
Gross Pathology: Multiple pale white streaks were seen on the epicardium. On cut surfaces of the heart, multiple irregular, pale white foci were observed in the myocardium, especially in the papillary muscle. Similar pale white streaks and irregular pale foci were noted in the skeletal muscles throughout the body, including the shoulders, brisket, quadriceps, and tongue.
A large abscess was found at the base of the umbilicus. All lung lobes were wet and heavy and slightly reddened. An increased lobular pattern was noted in the cranioventral lung lobes.
The entire length of the spinal cord was sectioned and examined after fixation. A demarcated, regionally extensive, brown focus of malacia was found in the lumbar spinal cord. Other significant gross abnormalities were not observed in the rest of spinal cord segments or the brain.
Laboratory results: Hepatic vitamin E level and trace mineral analysis (including selenium) were all within normal limits. Trueperella pyogenes was recovered from the umbilical abscess.
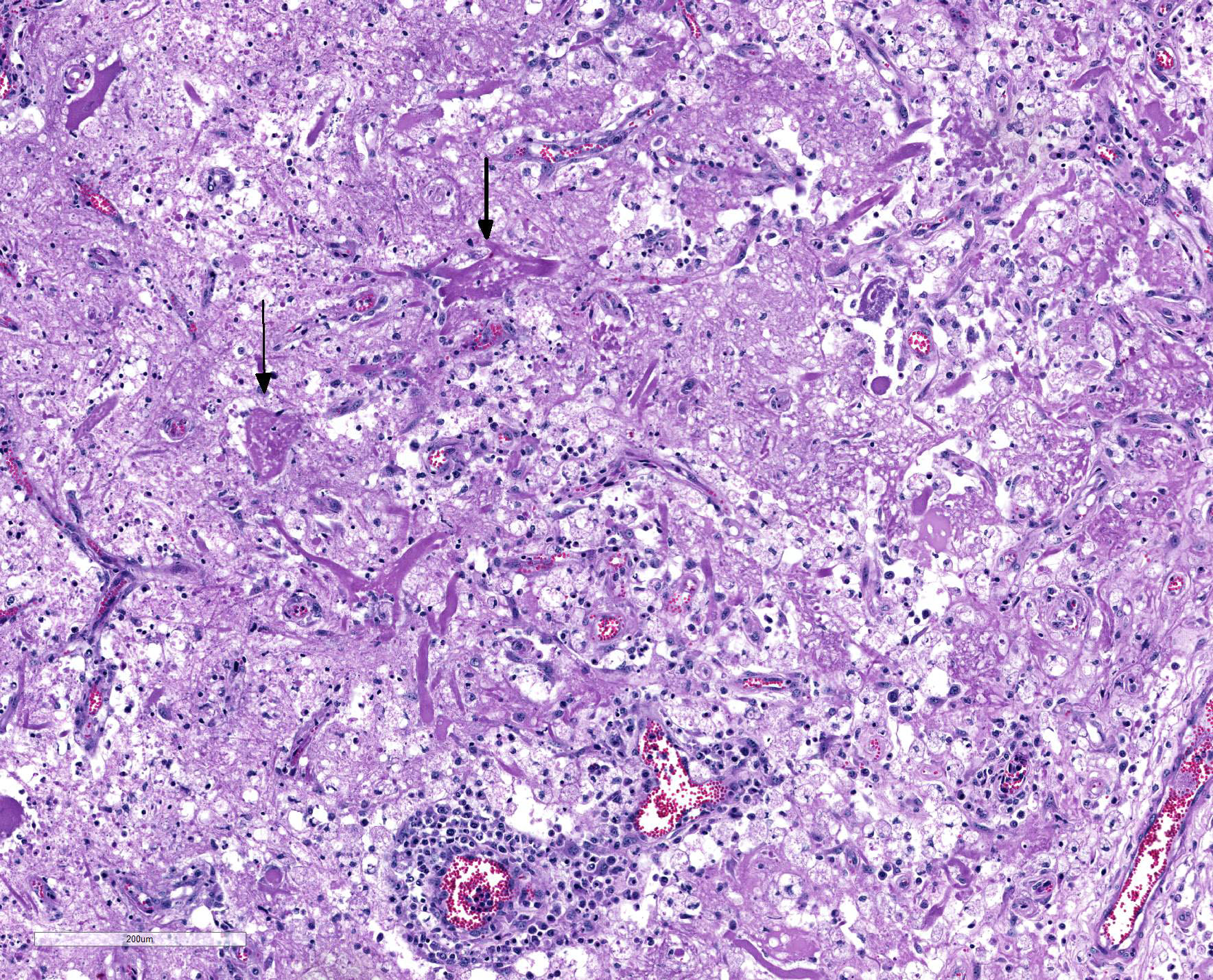
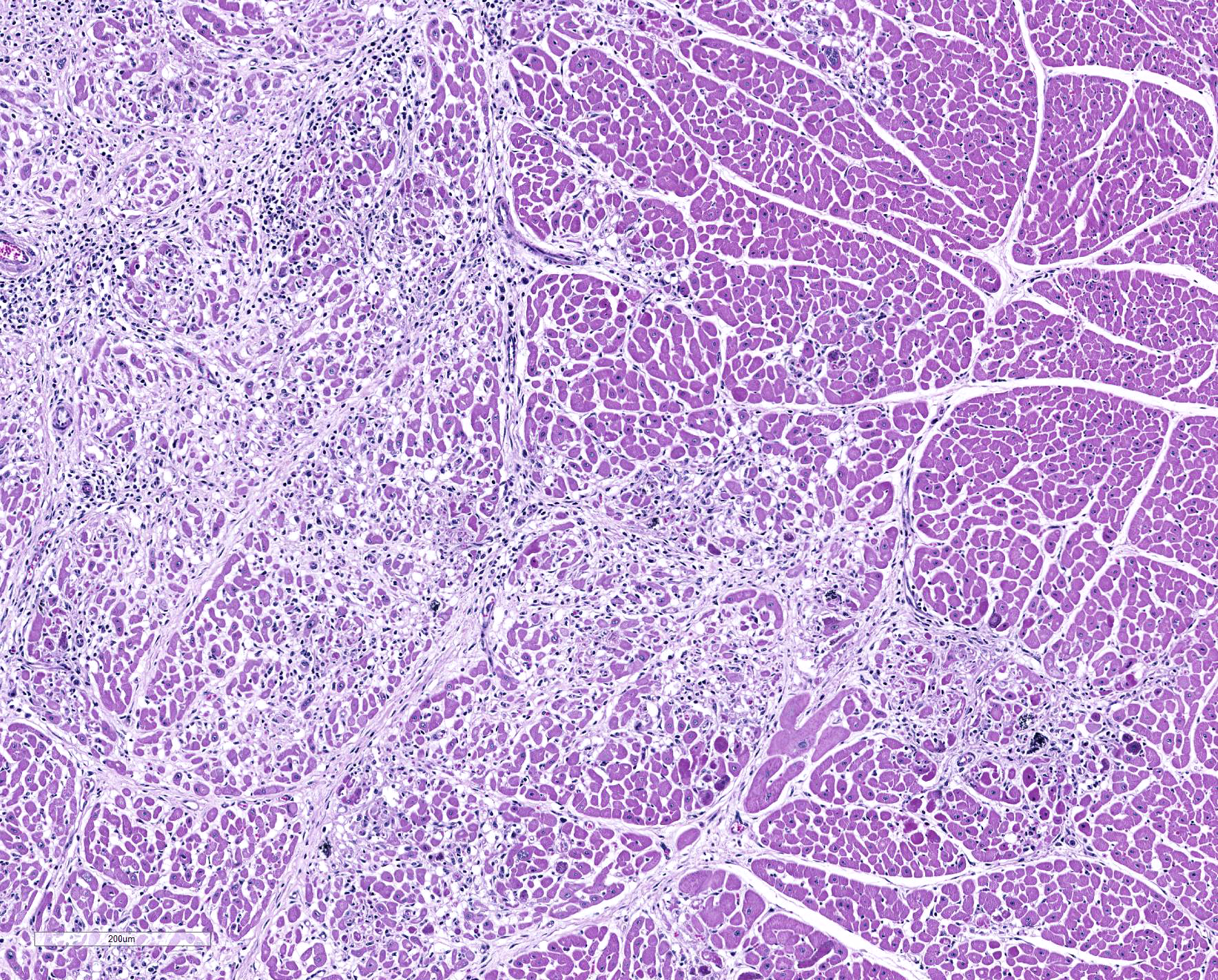
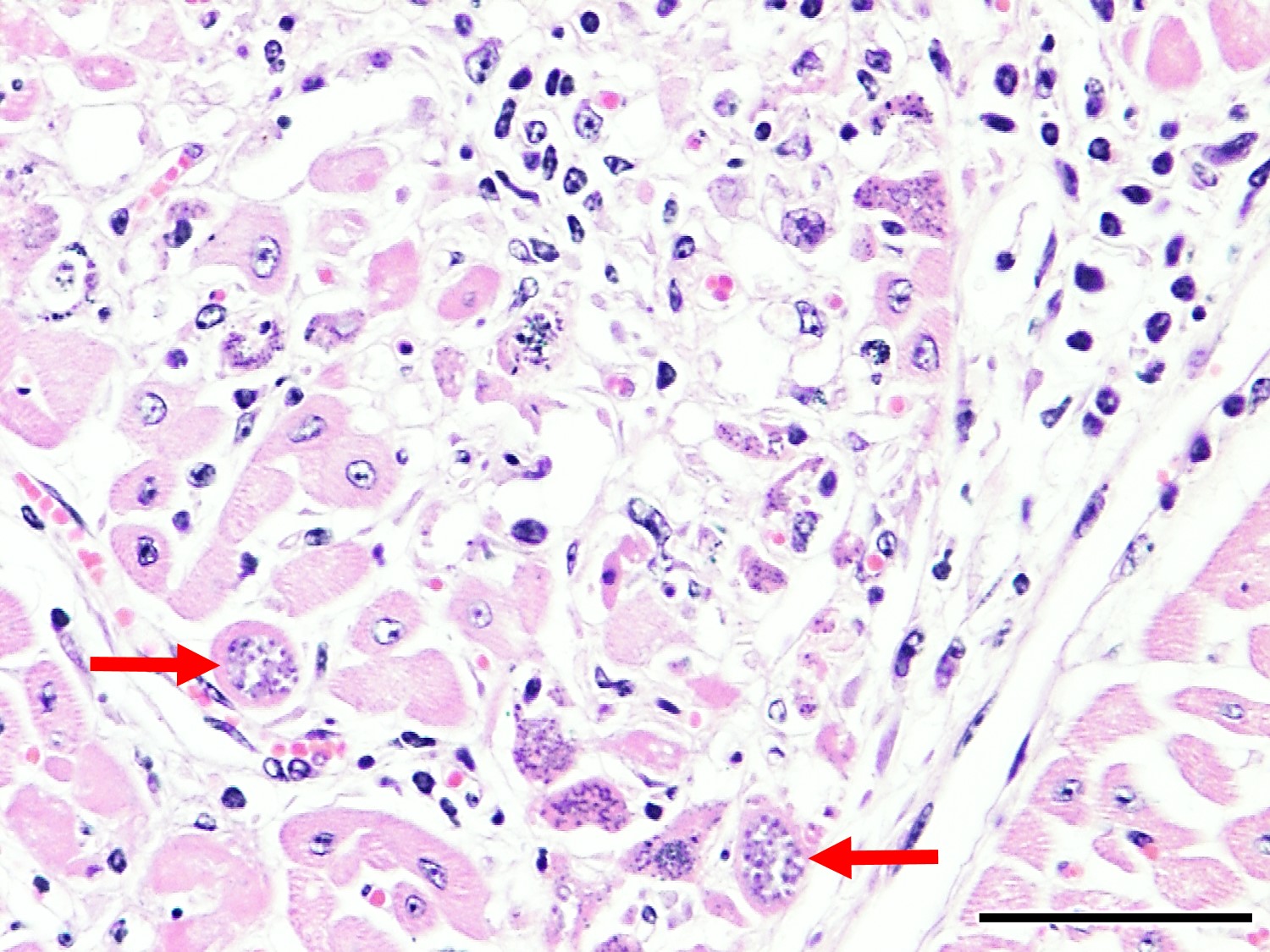
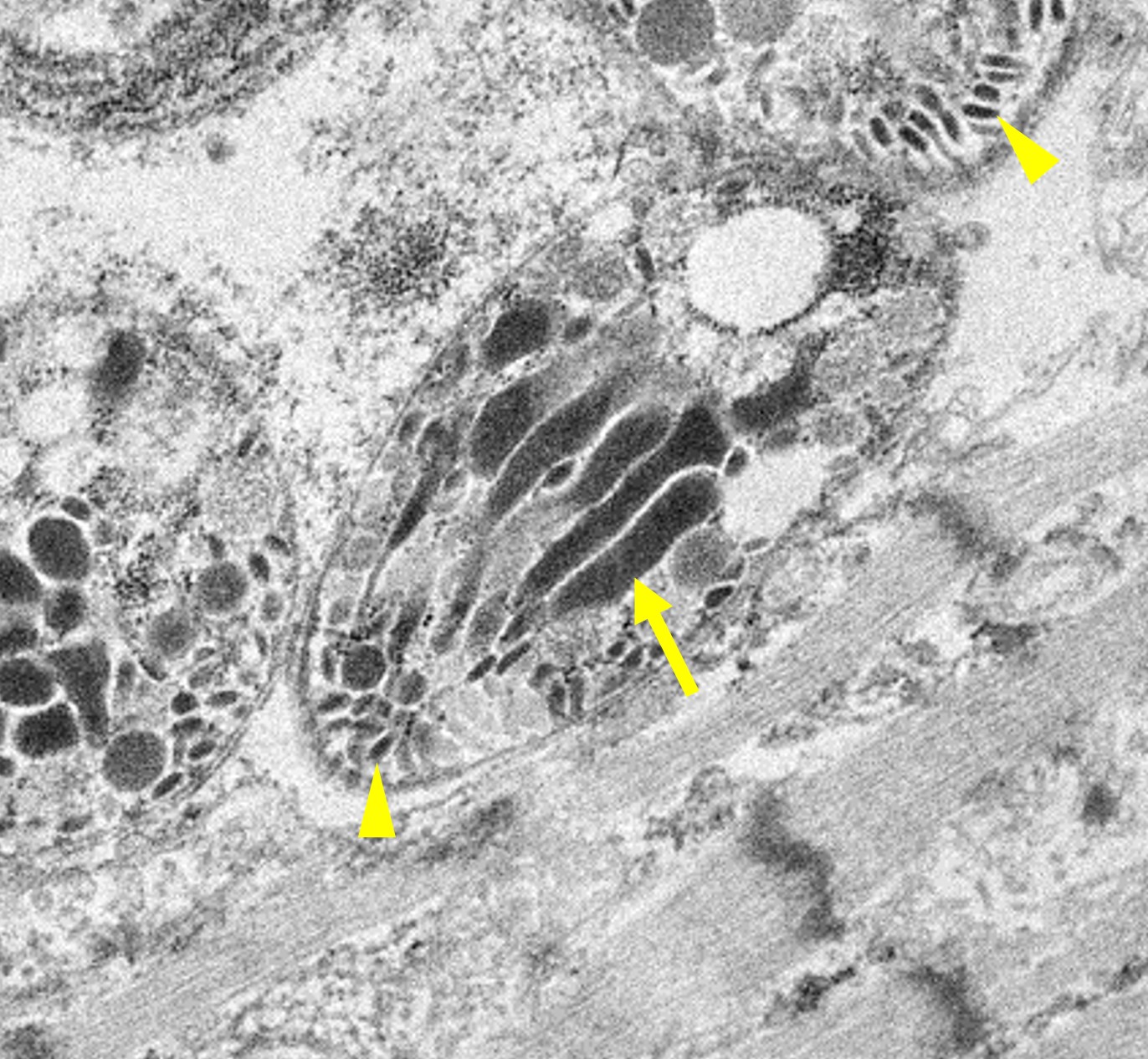
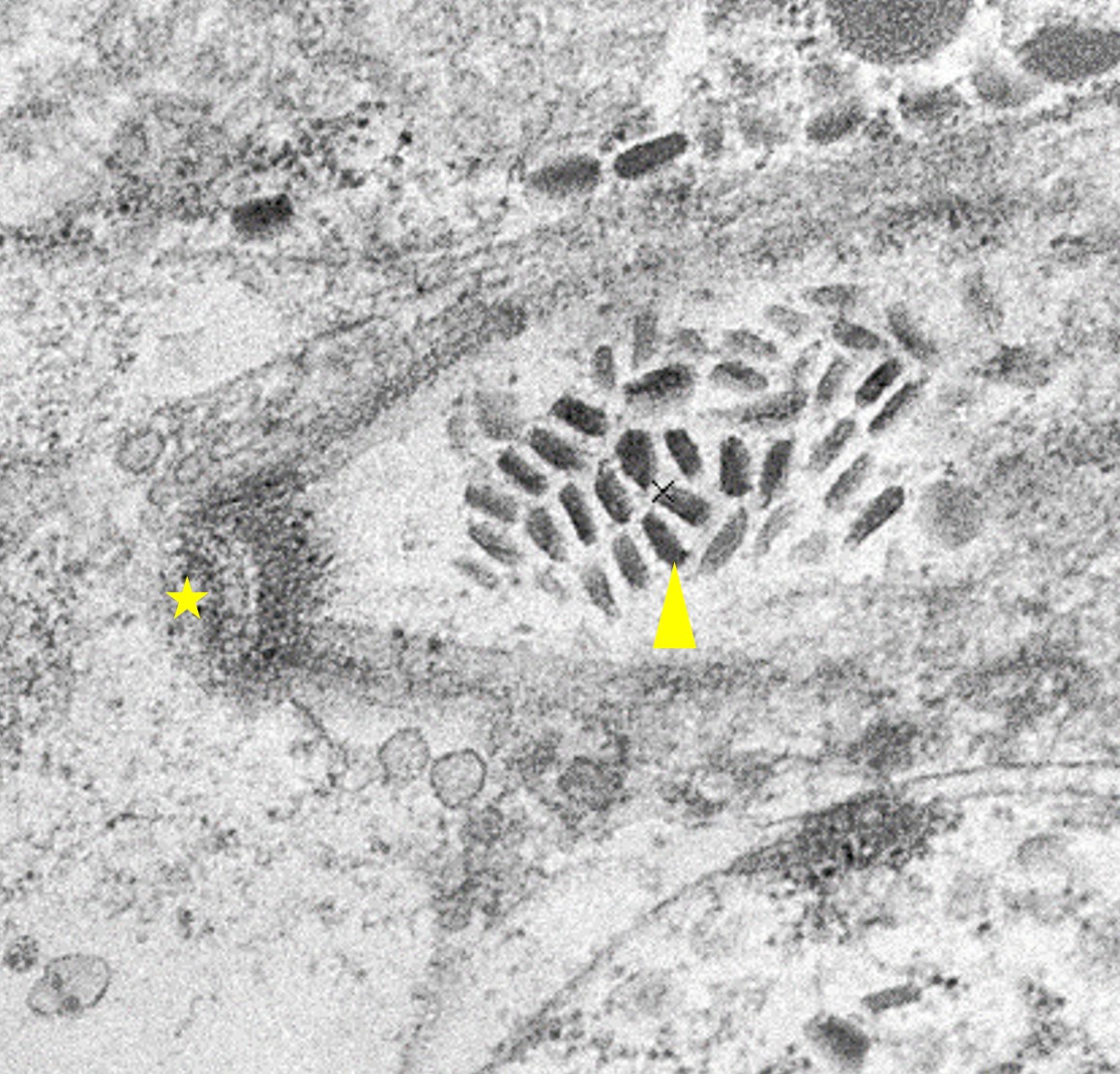
Microscopic Description: Heart: Multifocal to confluent necrotic foci are randomly distributed in the myocardium. Cardiomyocyte degeneration and necrosis characterized by hypereosinophilia, fragmentation, pyknosis, karyorrhexis, and mineralization is widespread. Loss of cardiomyocytes and collapse of endomysium are accompanied by fibrosis and infiltrates of lymphocytes, macrophages, and few neutrophils. Small clusters (10-50 µm in diameter) of intracytoplasmic protozoal tachyzoites are commonly found in the cardiomyocytes and occasionally in Purkinje fibers at the periphery of necrotic foci or non-inflamed areas. Tachyzoites are basophilic, crescent-shaped, approximately 5 x 2 µm, with a prominent central nucleus. Spherical protozoal tissue cysts, measuring 10-20 µm in diameter are uncommonly seen containing a thin (< 1 µm), eosinophilic cyst wall and numerous tightly packed bradyzoites. Multiple Anichkov cells are observed within areas of necrosis and fibrosis.
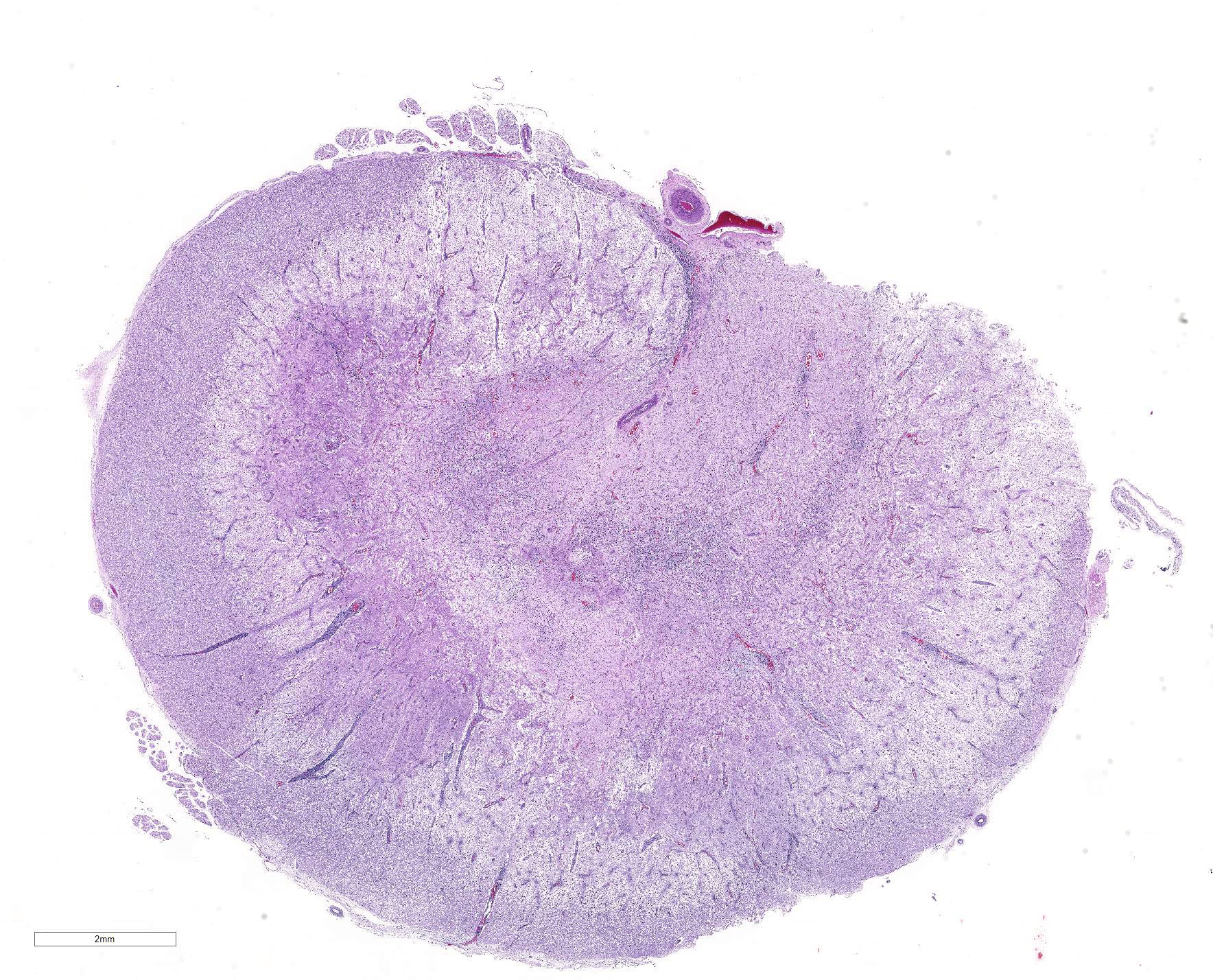
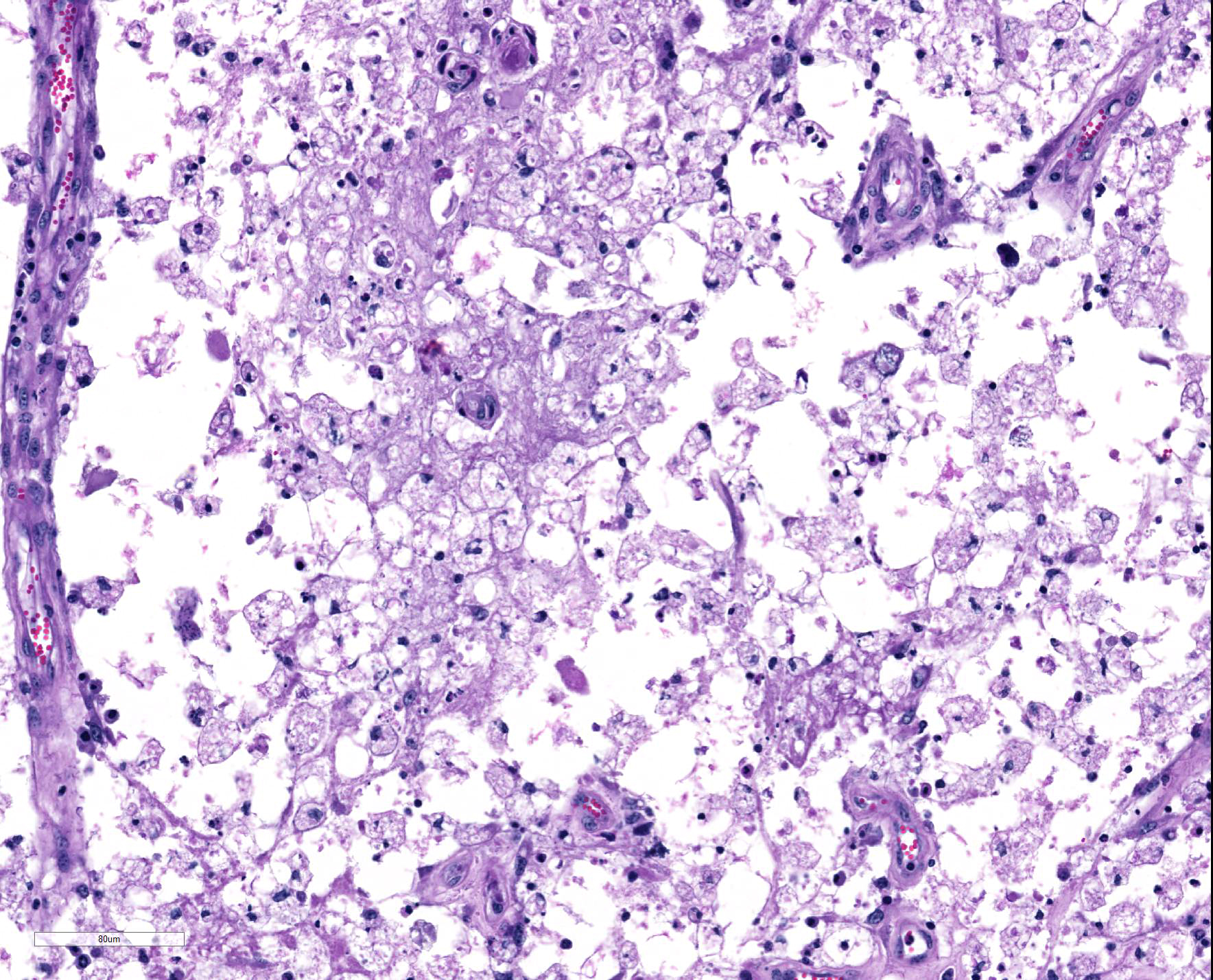
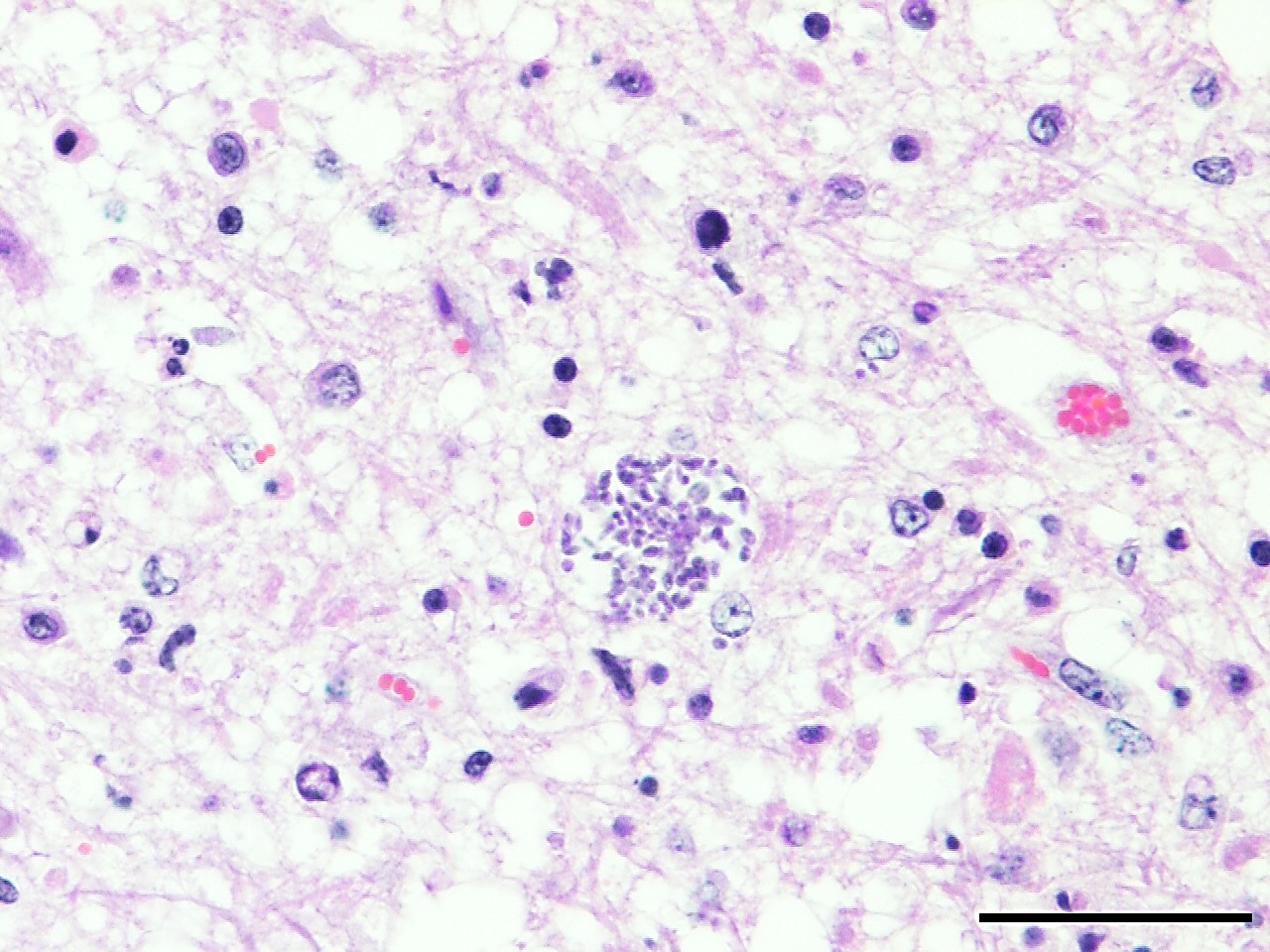
Spinal cord: In the lumbar spinal cord, more than 70% of the parenchyma is obscured by regionally extensive necrosis and accompanying inflammatory infiltrates. Lesions center on the ventral sulcus and the gray matter, extending to the white matter, and are characterized by marked spongiosis, disruption and loss of neuropil, neuronal degeneration and loss, replaced by numerous foamy gitter cells, foci of gliosis, and scattered lymphocytes and plasma cells. Clusters of protozoal tachyzoites and tissue cysts are commonly identified within the neuropil (Figs. 3 & 5) and vascular endothelium (Fig. 4), and occasionally also found within glial cells and neurons. Multifocally, Virchow-Robin spaces and the leptomeninges are expanded by moderate numbers (2-5 layers) of lymphocytes and neutrophils. Endothelial cells are hypertrophic, and occasionally contain intracytoplasmic protozoal tachyzoites. Axonal degeneration with spheroids is prominent. The central canal is disrupted by inflammation and filled with proteinaceous fluid, neutrophils, lymphocytes, cellular debris and intralesional tachyzoites. The lining ependymal cells are attenuated or necrotic and lost.
Contributor?s Morphologic Diagnosis:
Heart: Marked, chronic-active, multifocal to coalescing necrotizing myocarditis, lymphohistiocytic, with fibrosis, mineralization, abortive myocardial regeneration and intralesional protozoal tachyzoites and tissue cysts.
Lumbar spinal cord: Severe, subacute, multifocal and regionally extensive, necrotizing meningomyelitis, lymphohistiocytic and neutrophilic, with spongiosis, gliosis, perivascular cuffing, neuroaxonal degeneration and intralesional protozoal tachyzoites and tissue cysts.
Contributor?s Comment: The microscopic findings are consistent with apicomplexan protozoal infection-associated myocarditis and meningomyelitis. The causative protozoans are confirmed as Neospora caninum by their characteristic ultrastructural features. Similar lesions and microorganisms were also identified in the skeletal muscles throughout the body, and to a lesser extent, in the brainstem and the cerebrum of this calf.
Neosporosis is a significant cause of abortion and stillbirth in both beef and dairy cattle worldwide.1-5 The causative agent, Neospora caninum, is an apicomplexan protozoal parasite known as a major pathogen of cattle and dogs.1-3 Abortion may occur from 3 months gestation to term with a peak at 5-6 months gestation in the affected cows.2 Other than reproductive failure, N. caninum infection rarely causes clinical disease in adult cattle and calves.1 However, occasionally, neurologic disease as a result of encephalomyelitis may occur in congenitally infected calves less than 4 months of age2 and clinical signs such as ataxia, hyperextension of limbs, weakness and paralysis have been reported.2,3,6,7,8
Dogs and several wild canids are the definitive hosts of N. caninum, whereas cattle and other warm-blooded animals serve as intermediate hosts.1,5 The pathogenesis of N. caninum infection in cattle is not fully understood. Both transplacental and horizontal transmissions play an important role in bovine neosporosis.1,6 Transplacental transmission occurs when tissue cysts are reactivated in the persistently infected cow (endogenous) or when the oocysts are ingested by the naïve cow (exogenous).1,6 N. caninum is transmitted efficiently from the pregnant cows to their offspring during pregnancy, and the consequences of infection include abortion, birth of a weak calf occasionally with neurologic signs, or birth of a persistently infected but clinically healthy calf.1,3 Abortion is believed to be a result of one or more of the following mechanisms: (1) primary parasite-induced placental damage; (2) fetal tissue damage due to parasite multiplication; (3) maternal immune expulsion of the fetus (Th1-type immunoresponse) due to N. caninum induced pro-inflammatory cytokines in the placenta.1,3 Cow-to-cow transmission has not yet been proven.1 The only demonstrated natural mode of postnatal infection in cattle is ingestion of sporulated N. caninum oocysts from the environment (e.g. contaminated canine feces).9
Neospora caninum is an intracellular protozoa and has three infectious stages, including sporozoites, tachyzoites and bradyzoites.1 Sporozoites are present in oocysts that are shed in the feces of the definitive host. Tachyzoites and bradyzoites are found in the tissues of both intermediate and definitive hosts.1-3 Tachyzoites are rapidly dividing forms, crescent-shaped, approximately 6 x 2 µm with a centrally placed nucleus. They may infect a variety of cells including neural cells, vascular endothelial cells, myocytes, hepatocytes, renal cells, alveolar macrophages and placental trophoblasts.1 Bradyzoites are slowly replicating forms present within the tissue cysts. They are slender, approximately 6.5 x 1.5 µm with a terminally located nucleus and contain several Periodic Acid Schiff positive amylopectin granules.1 Tissue cysts found in infected calves are usually smaller (<50 µm in diameter) with thinner (<2 µm thick) walls compared to those in dogs (up to 107 µm in diameter).1
In the current case, the lesions were most severe in the lumbar spinal cord, heart, and the skeletal muscles. Interestingly, the gross lesions somewhat resembled those of calves with vitamin E/selenium deficiency (white muscle disease), because of the widespread myonecrosis. However, neosporosis was confirmed on histopathology by demonstrating numerous N. caninum organisms. Minor lesions were also noted in the brainstem and cerebrum, characterized by foci of necrosis with gliosis, aggregates of mononuclear cells and prominent perivascular cuffing. Furthermore, the vitamin E and selenium levels were within normal limits in this calf.
Microscopically, abortifacient apicomplexan protozoans such as N. caninum, Toxoplasma gondii, and Sarcocystis cruzi, can be indistinguishable in HE stained sections.2,5 Toxoplasma gondii is seen in rodents, dogs, cats, and human with CNS and reproductive diseases but it has not been proven to cause abortion in cattle.2 A recent study documented that some species of Sarcocystis, specifically S. cruzi, can cause neurologic disease in calves and adult cattle.10 However, it was suggested that if apicomplexan-like protozoans are seen in the brain tissue of aborted calves, they can be assumed to be N. caninum.2
Ultrastructurally, N. caninum, T. gondii, and S. cruzi have different morphologies that can be distinguished from each other.2,11 N. caninum contains 8-12 electron-dense rhoptries and numerous micronemes cranial to the nucleus, whereas T. gondii contains few (4-8) spongy or honeycomb-like rhoptries, few micronemes, and many micropores.11 Individual merozoites of S. cruzi are smaller (3-5 x 2-3 µm) compared to the others and they have numerous micronemes but lack rhoptries.2,10
Immunohistochemistry staining is a useful tool to identify N. caninum organisms on histopathology slides, but false positives due to cross reactions to T. gondii have been reported in some studies.2,12 PCR is another commonly used method for diagnosis and many PCR assays targeting different N. caninum genes have been published.2,12 Serology is a practical tool to diagnose N. caninum-related abortions. It has an advantage of antemortem diagnosis of the disease and is commonly used as a screening test in herds.2
Contributing Institution:
Oklahoma State University
Department of Veterinary Pathobiology
College of Veterinary Medicine
250 McElroy Hall
Stillwater, OK 74078
https://cvhs.okstate.edu/Veterinary_Pathobiology
JPC Diagnosis: 1. Spinal cord, grey and white matter: Myelitis, necrotizing, multifocal to coalescing, severe with mild multifocal lymphohistiocytic and neutrophilic meningitis, and numerous intracellular apicomplexan zoites.
2. Heart: Myocarditis, necrotizing, subacute, multifocal to coalescing, marked, with occasional intracellular apicomplexan zoites.
JPC
Comment: The
contributor has provided an excellent review of Neospora, a common
finding in bovine abortion in many parts of the US. The comment is so
well-constructed, that our comment in turn will focus on some lesser known, but
interesting "facts" about Neospora that are often overlooked in its
review.
While not an author on the original Norwegian paper by Bjerkas et al. describing Neospora as an "unidentified cyst-forming sporozoon" causing encephalomyelitis and myositis in three consecutive litters of dogs, a PubMed search for both "Neospora" and "Dubey" brings up an amazing 277 titles.
In the last thirty years, Neospora caninum has appeared eleven times in the Wednesday Slide Conference, (and a twelfth submission was diagnosed as Sarcocystis by Dr. J.P. Dubey based on ultrastructural analysis and later, immunohistological confirmation). In the WSC, it has actually been submitted more often in the dog, from the brain (4), spinal cord (3), skin (2) and skeletal muscle (1). In the ox, this will be the second case in the spinal cord, and the first submission of the heart lesion; a previously presented case was diagnosed in the brainstem.
In addition to the very characteristic infections in intermediate hosts such as cattle and dogs (dogs also serve as the definitve host), it has also been found in aberrant hosts (usually singular cases) to include sheep, water buffalo, horses, goats, white-tailed deer, a raccoon, and a rhinoceros. Dr. Dubey stresses that simply finding DNA of N. caninum, or antibodies to the organism, is not synonymous with identiifying the viable apicomplexan parasite histologically. Serologic positivity to N. caninum has been well documented in humans, but disease has not.13
Dubey et al
considers N. caninum to be one of the most ?efficiently transplacentally
transmitted parasites among all known microbes in cattle,? with all calves in
some herds being born infected but asymptomatic.13 In some parts of
the US, it is such a common cause of abortion in calves that, even in the
absence of finding characteristic tissue cysts, the presence of glial nodules
in the brain and areas of necrosis in the heart or liver is considered adequate
proof of its etiology. Cow-to-cow (horizontal) transmission has not been
established.14 While previously identified in the CNS of adult
horses, a recent publication documents the presents of Neospora
tachyzoites in the lung, liver and heart of an equine abortus, suggesting that N.
caninum should now be considered as an uncommon cause of equine abortion as
well. 15
References:
1. Dubey JP, Buxton D, Wouda W. Pathogenesis of bovine neosporosis. J Comp Pathol. 2006;134(4):267-289.
2. Dubey JP, Schares G. Diagnosis of bovine neosporosis. Vet Parasitol. 2006;140(1-2):1-34.
3. Innes EA, Wright S, Bartley P, et al. The host-parasite relationship in bovine neosporosis. Vet Immunol Immunopathol. 2005;108(1-2):29-36.
4. Helman RG, Stair EL, Lehenbauer TW, et al. Neosporal abortion in Oklahoma cattle with emphasis on the distribution of brain lesions in aborted fetuses. J Vet Diagn Invest. 1998;10(3):292-295.
5. Dubey JP, Schares G. Neosporosis in animals--the last five years. Vet Parasitol. 2011;180(1-2):90-108.
6. Marugan-Hernandez V. Neospora caninum and Bovine Neosporosis: Current Vaccine Research. J Comp Pathol. 2017;157(2-3):193-200.
7. Uesaka K, Koyama K, Horiuchi N, et al. A clinical case of neosporosis in a 4-week-old holstein friesian calf which developed hindlimb paresis postnatally. J Vet Med Sci. 2018;80(2):280-283.
8. De Meerschman F, Focant C, Detry J, et al. Clinical, pathological and diagnostic aspects of congenital neosporosis in a series of naturally infected calves. Vet Rec. 2005;157(4):115-118.
9. McCann CM, McAllister MM, Gondim LFP, et al. Neospora caninum in cattle: Experimental infection with oocysts can result in exogenous transplacental infection, but not endogenous transplacental infection in the subsequent pregnancy. International Journal for Parasitology. 2007;37(14):1631-1639.
10. Dubey JP, Calero-Bernal R, Verma SK, et al. Pathology, immunohistochemistry, and ultrastructural findings associated with neurological sarcocystosis in cattle. Vet Parasitol. 2016;223:147-152.
11. Lindsay DS, Speer CA, Toivio-Kinnucan MA, et al. Use of infected cultured cells to compare ultrastructural features of Neospora caninum from dogs and Toxoplasma gondii. Am J Vet Res. 1993;54(1):103-106.
12. van Maanen C, Wouda W, Schares G, et al. An interlaboratory comparison of immunohistochemistry and PCR methods for detection of Neospora caninum in bovine foetal tissues. Vet Parasitol. 2004;126(4):351-364.
13. Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 2007; 20(2):323-367.
14. Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasit 2003; 41(1):1-16.
15. Anderson JA, Alves DA, Cerquieira-Cezar CK, da Silva AF, Murata FHA, Norris JK, Howe DK, Dubey JP. Histologically, immunohistochemically, ultrastructurally, and molecularly confirmed neosporosis abortion in an aborted equine fetus. Vet Parasit 2019; 270:20-24.