Signalment:
Gross Description:
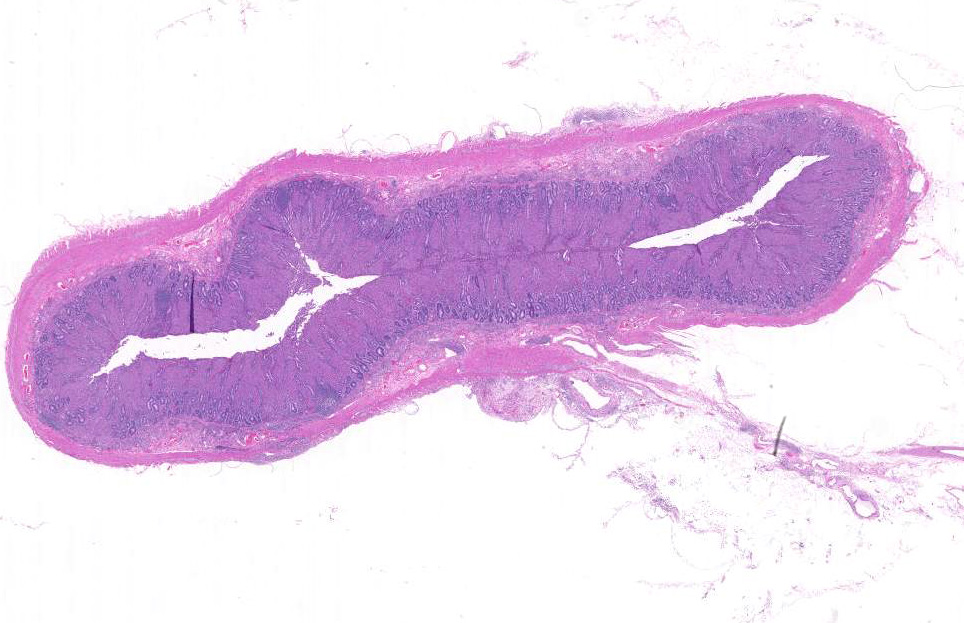
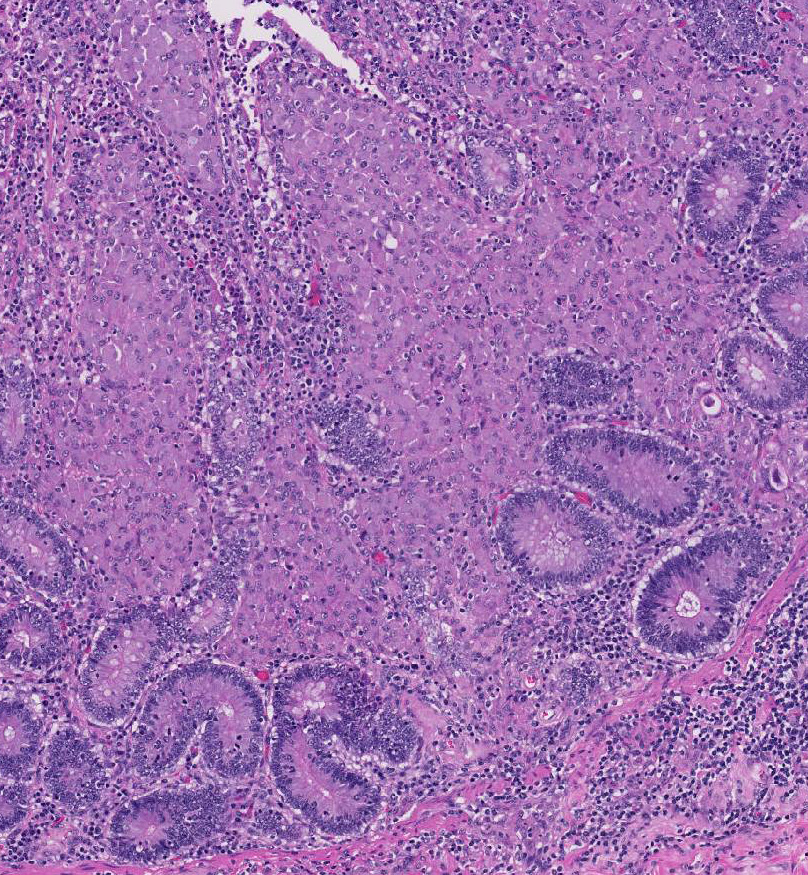
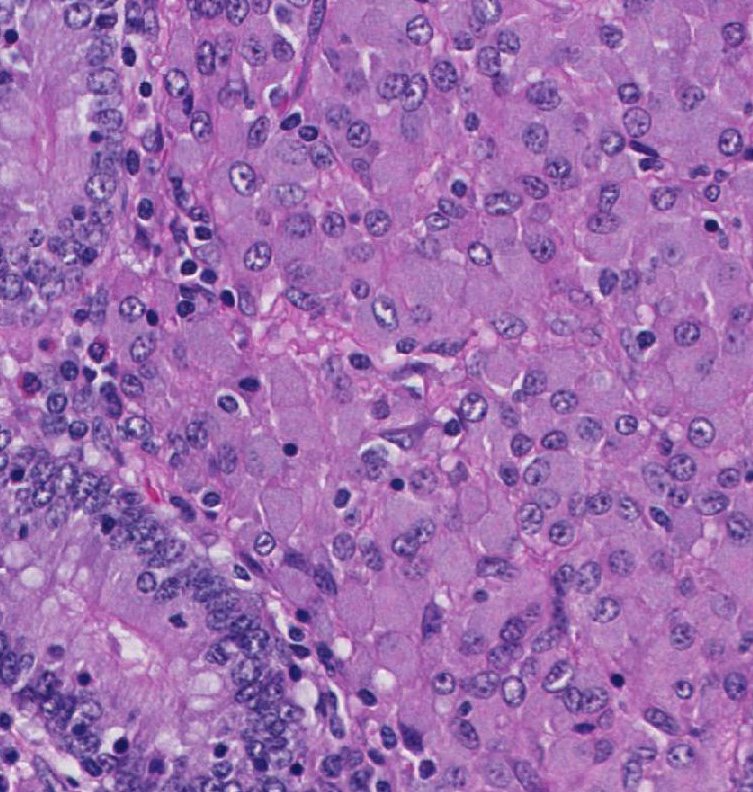
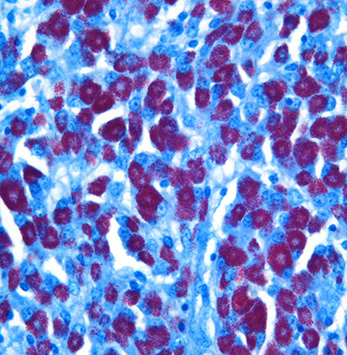
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Sheep are usually infected early in life via the fecal-oral route, although infection can also be acquired through consumption of contaminated colostrum or in utero during advanced stages of the disease. A recent study characterized the pathology following experimental infection of MAP in adult sheep and found focal lesions restricted to the intestinal lymphoid tissue.2 The M cells, specialized non-villous epithelial cells located in the Peyers patches of small intestine, act as a main portal of entry and facilitate translocation of MAP across the intestinal epithelium where they are phagocytized by macrophages. Macrophage recognition of MAP is mediated by host pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs) and intracellular NOD-like receptors (NLRs). The MAP bacterium has a unique ability to replicate within macrophages by blocking or modulating antimicrobial activities that allow long-term survival.5
Broadly, two distinct phenotypes have been recognized based on the histologic features and the pathogen load: the lepromatous (organism-rich or multibacillary) form and the tuberculoid (paucibacillary) form. It is generally accepted that animals with paucibacillary lesions have a dominant Th1 type (IFN-γ) immune response, while animals with multibacillary lesions tend to have predominant Th2 type (antibody) response.8 The progression in the severity of disease and degree of intestinal bacterial load parallels a switch from Th1 to a Th2 response.3 In the present case, the ileum showed diffuse multibacillary feature (Type 3b) according to well-established histological criteria for classifying lesions associated with natural paratuberculosis in sheep.6
Although the pathogenesis of Johnes disease in small ruminants is generally accepted to be similar to that in cattle, the clinical manifestations and gross pathology tend to be more insidious. Affected sheep show poor quality fleece, chronic wasting, and submandibular edema secondary to hypoalbuminemia, but overt diarrhea is not a common feature unlike in cattle. Enteric gross lesions are mild with little obvious thickening and no transverse ridges. Mineralized tubercle-like lesions in the mucosa, submucosa, serosa, and lymphatics of the intestine or lymph nodes have also been reported in goats and occasionally in sheep.9 The diagnosis of Johnes disease is difficult because of the organisms fastidious growth requirement and the lack of a specific diagnostic test that is sensitive enough to detect subclinical animals. Traditionally, fecal culture for MAP is considered the gold standard for diagnosis but is timeconsuming and has poor reliability. As the humoral immune response is elicited late during infection, serological tests such as enzyme-linked immunosorbent assay (ELISA) are even less sensitive than fecal culture. The use of PCR for detection of MAP by amplifying the IS900 gene sequence in fecal samples has vastly improved the detection of low shedders.10 Vaccination can be useful, but current vaccines have significant drawbacks that prevent their widespread use.
JPC Diagnosis:
Conference Comment:
The focal form of MAP infection is typified by small, well-demarcated, granulomas in the lymphatic tissue of the intestine and lymph nodes; these are characteristic for the initial and latent stages of infection in adult animals. Multifocal forms are present in subclinical infections, and animals manifest with small granulomas in lymphoid tissue and in the lamina propria of the intestine; however, the normal histological architecture of the intestine is not drastically changed. This multifocal form is often subclinical and affected animals can still shed the organism and be infectious to herd mates resulting in the iceberg effect, because in an infected herd, only a small percentage of the animals demonstrate clinical signs. Sheep and goats are generally thought to be more susceptible to clinical disease and have a shorter incubation period.2,3,4,9,11
Diffuse lesions are characterized by generalized granulomatous enteritis that affects both lymphoid tissue and the lamina propria of the intestine. This form, unlike the multifocal form, causes characteristic widespread cerebriform thickening of the intestinal wall. The diffuse form is divided into two categories based on the cell types and numbers of acid-fast bacteria (AFB) present: 1) the multibacillary/histiocytic form is composed of sheets of epithelioid macrophages with large numbers of intracytoplasmic AFB; and 2) the paucibacillary/lymphocytic form is typified by numerous lymphocytes within the lamina propria surrounding scattered granulomas with macrophages containing few or no AFB.
The focal, multifocal, or diffuse paucibacillary lesions are associated with the tuberculoid form mentioned by the contributor, and are a consequence of host bias toward a cell-mediated immunity response, with predominance of M1 or classically activated macrophages. Classically activated macrophages are more effective at killing intracellular bacteria. The diffuse multibacillary lepromatous form, present in this case, is associated with the less effective humoral response and M2, or alternatively activated, macrophages.4,9,11 The virulence factors expressed by mycobacterial agents play a major role in the lesion type and pathogenicity of the organism. A recent study identified the macrophage subsets within granulomatous lesions in bovine paratuberculosis,4 and as mentioned by the contributor, the pathogenesis in sheep is likely similar to that of cattle. The MAP organism, like other mycobacterial agents, does not secrete toxins; instead, its virulence is based on properties of its cell wall. Mannose and CD14 receptors expressed on macrophages stimulate phagocytosis of the organism. After phagocytosis, the mycobacterial cell wall receptor lipoarabinomannan (LAM) inhibits macrophage phagosome maturation by inducing expression of cytokines IL-10 and TGF-β through activation of TLR2 in the phagosome.4,9,11 The bacterium can also inhibit acidification of the phagosome and phagosome-lysosome fusion. The cytokine IL-10 suppresses M1 macrophage activation, and thus the cell- mediated response, and enhances the Th2 humoral response. Further recruitment of alternatively activated M2 macrophages leads to widespread infection and progressive inflammation.4,9,11 As the severity of the inflammation increases, MAP secretes exochelins, iron reductases, and siderophores to acquire iron from ferritin stored in macrophages, and inhibits iron-dependent conversion of H2O2 into hydroxyl radicals via the Fenton reaction.11
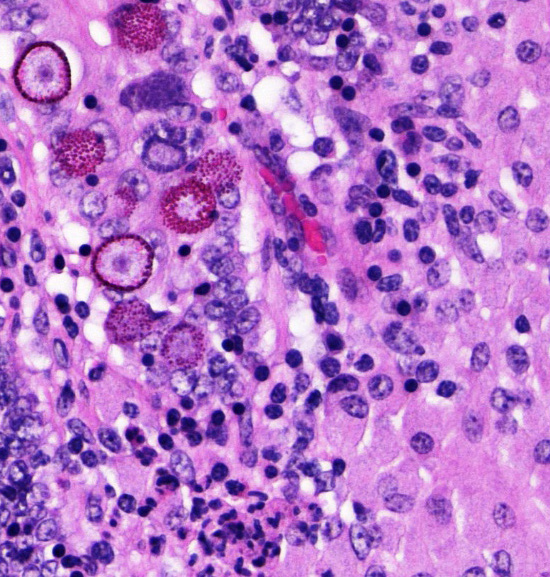
Several conference participants also noted rare intraepithelial coccidian microgamonts, macrogamonts, schizonts, and oocysts within the intestinal crypts. In sheep, the likely causes of coccidioisis in the small intestine are Eimeria christenseni, E. ahsata, E. brakuensis, and E. crandallis. In the cecum and colon of sheep, the most likely cause is E. ovinoidalis.9
References:
2. Delgado L, Marin JF, Munoz M, et al. Pathological findings in young and adult sheep following experimental infection with 2 different doses of Mycobacterium avium subspecies paratuberculosis. Vet Pathol. 2013;50(5):857-866. Intestine, sheep. An acid-fast stain on the mucosal infiltrate demonstrates large numbers of intracytoplasmic acid-fast bacilli. (AF, 400X) (Photo courtesy of: Department of Diagnostic Medicine and Pathobiology, Kansas State Veterinary College of Veterinary Medicine, 1800 Denison Avenue, Manhattan, KS 66506, http://www.vet.k-state.edu/depts/dmp/index.htm ) 6
3. Dennis MM, Reddacliff LA, Whittington RJ. Longitudinal study of clinicopathological features of Johne's disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Vet Pathol. 2011;48(3):565-575.
4. Fernandez M, Benavides J, et al. Macrophage subsets within granulomatous intestinal lesions in Bovine paratuberculosis. Vet Pathol. 2016; Jun 17: pii: 0300985816653794. [Epub ahead of print].
5. Koets AP, Eda S, Sreevatsan S. The within host dynamics of Mycobacterium avium ssp. Paratuberculosis infection in cattle: where time and place matter. Vet Res. 2015;46(1):1-17.
6. Perez V, Marin JG, Badiola J. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J Comp Pathol. 1996;114(2):107-122.
7. Robbe-Austerman S. Control of paratuberculosis in small ruminants. Veterinary Clinics of North America: Food Animal Practice. 2011;27(3):609-620.
8. Sweeney RW. Pathogenesis of paratuberculosis. Veterinary Clinics of North America: Food Animal Practice. 2011;27(3):537-546.
9. Uzal F, Plattner B, Hostetter J. Alimentary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Limited; 2016: 194-197.
10. Windsor P. Paratuberculosis in sheep and goats. Vet Microbiol. 2015;181(1):161-169.
11. Zachary JF. Mechanisms of microbial infections. In: McGavin MD, Zachary JF, ed. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:172- 173.