Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 25
1 May 2019
CASE I: N261/13 (JPC 4037902)
Signalment: 5 month old, female, Devon rex, Felis domesticus, feline.
History: This young, fully vaccinated, pedigree Devon rex cat with no previous history of illness became acutely ill with pyrexia and severe dyspnea, the morning following its attendance at a cat show where it had won both classes of competition for which it had been entered. Despite immediate veterinary attention and antibiotic treatment, the animal died within 36 hrs and was submitted for necropsy.
Gross Pathology: The carcass is well preserved with adequate body fat reserves. Periocular and perinasal regions are encrusted with light brown discharge. Tracheal and bronchial lumens filled with frothy mucopurulent exudate. There is multifocal consolidation in all lung lobes: on sectioning coalescing pale firm foci are present. The kidneys are bilaterally congested at the cortico-medullary junction. The stomach contains mucus only. Feces of normal color and consistency in rectum. The urinary bladder is empty.
Laboratory results: Both Bordetella bronchiseptica and Mycoplasma felis were cultured from samples of lung.
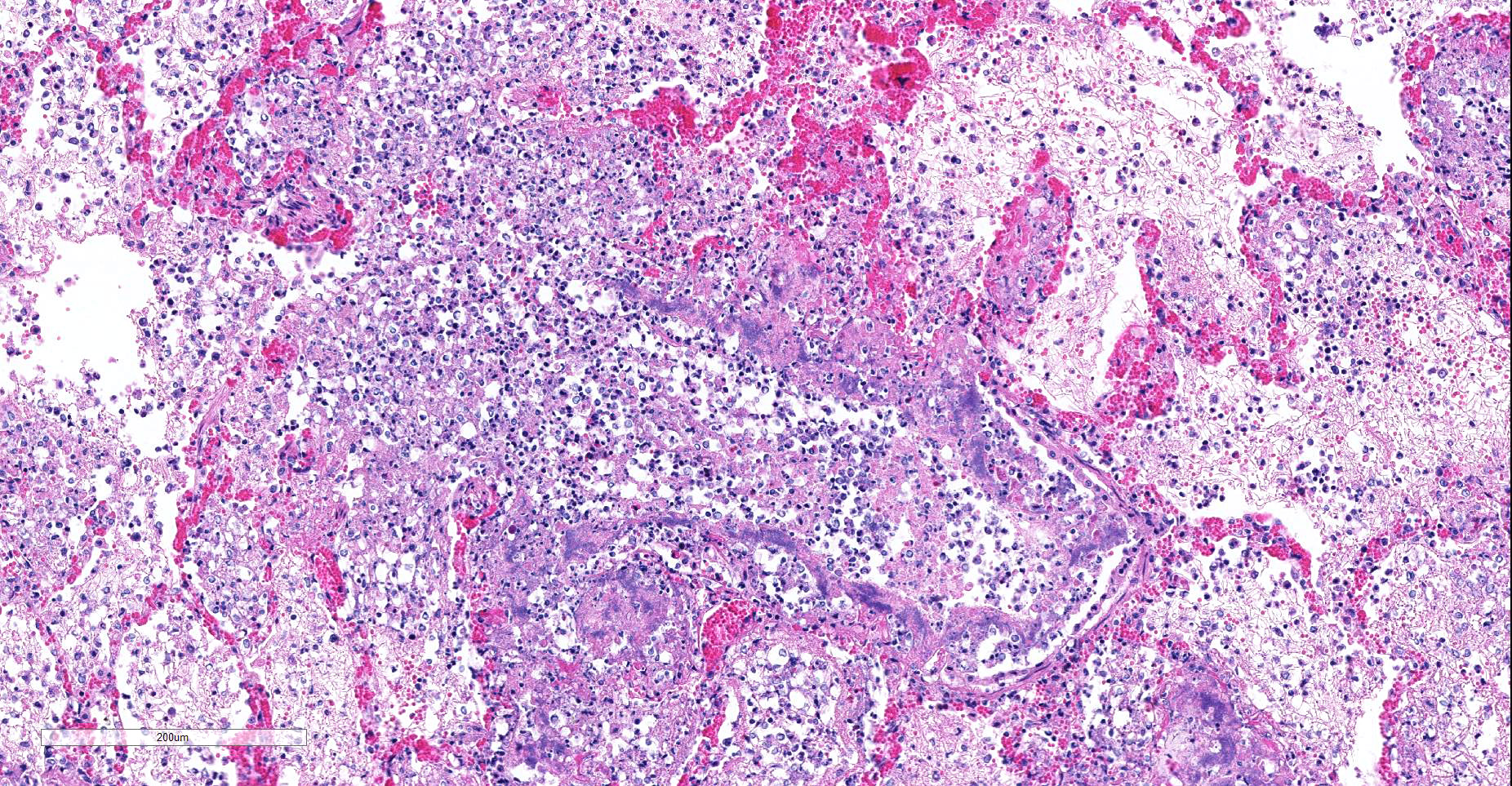
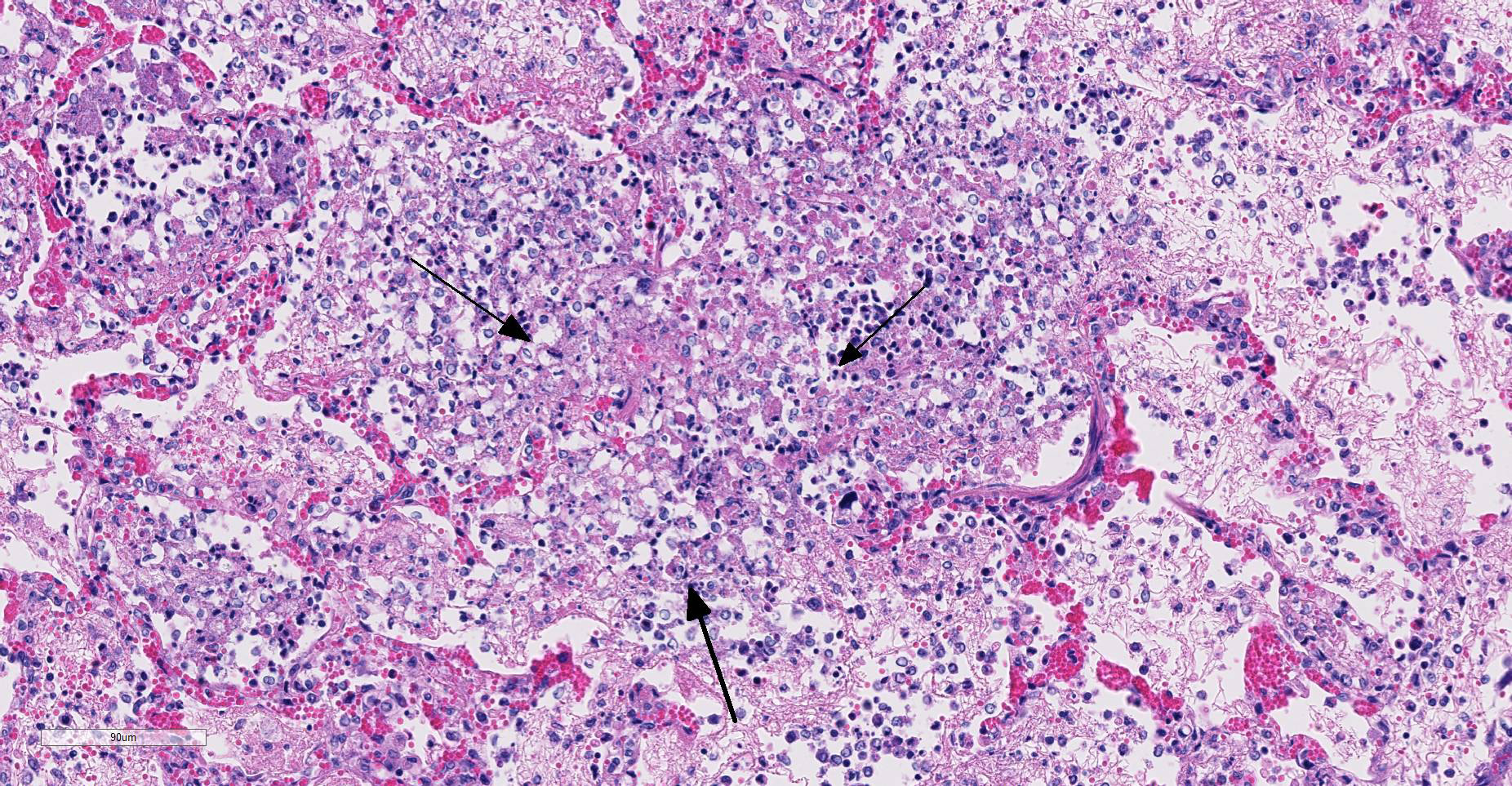
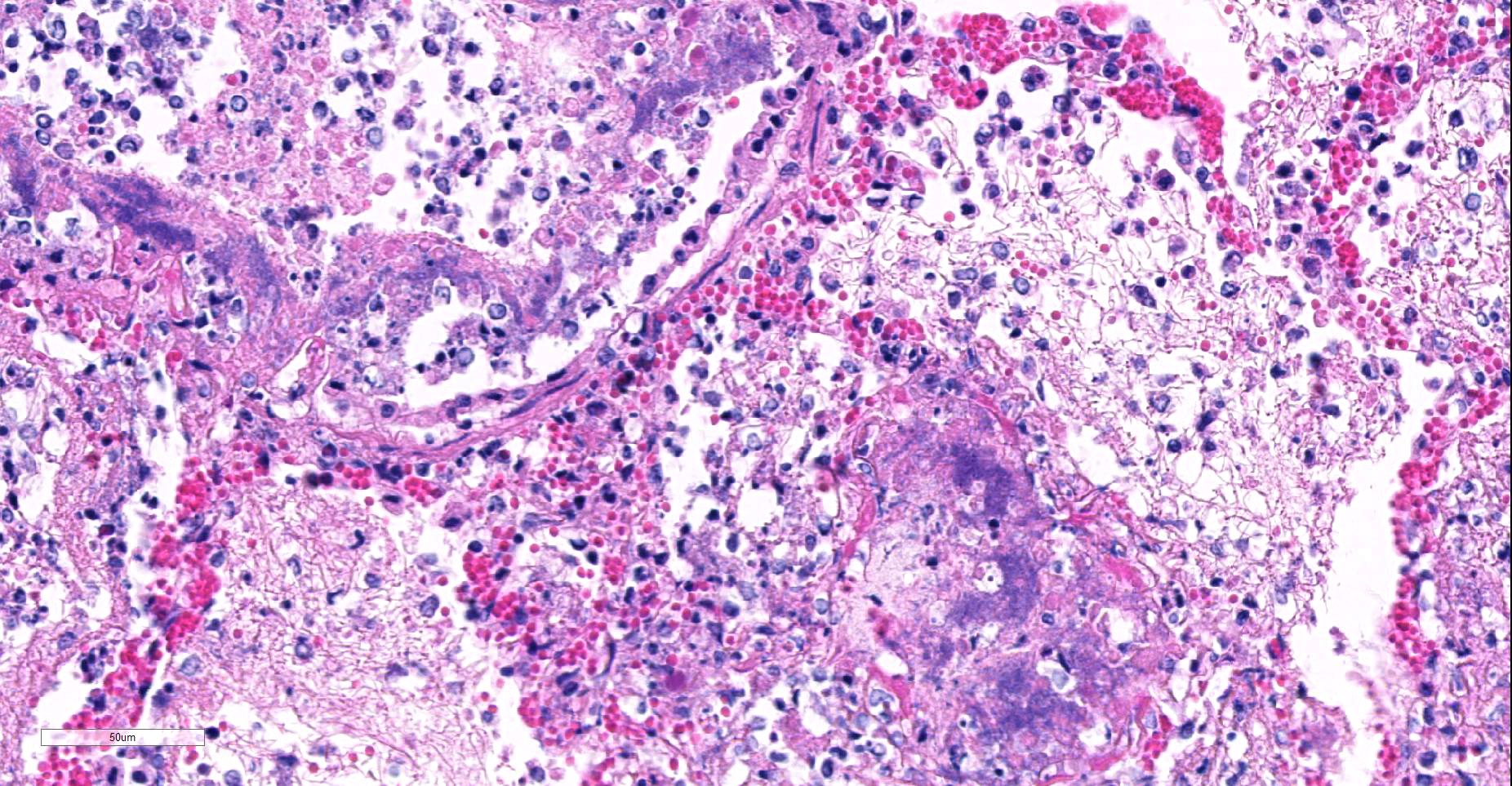
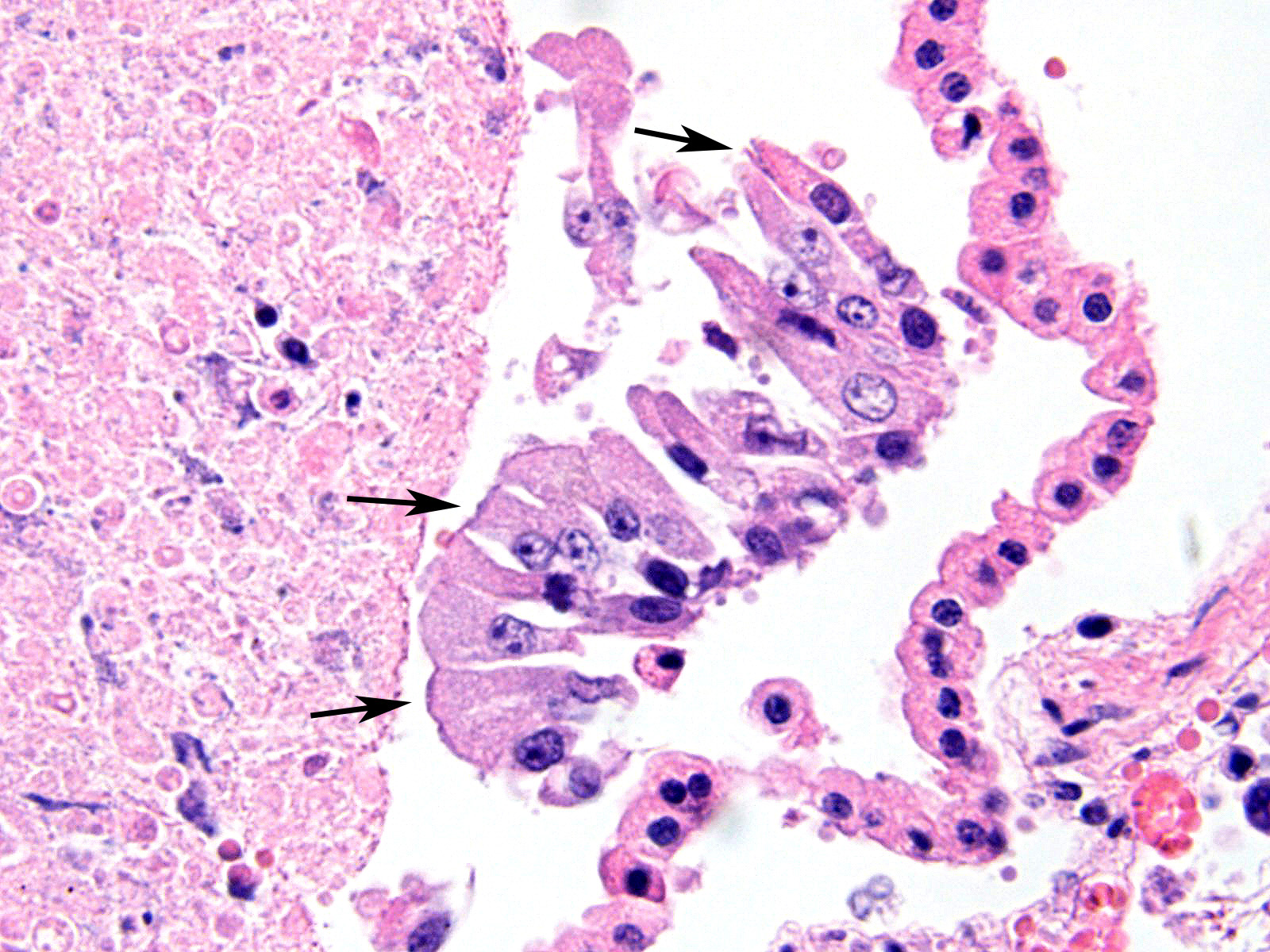
Microscopic Description: Multifocally, there is extensive necrosis of contiguous alveolar walls with filling of associated airspace with cell debris, protein-rich fluid/fibrin, and admixed intact and degenerate inflammatory cells (predominantly neutrophils with fewer macrophages and lymphocytes). Myriad loosely scattered to clumped basophilic coccobacilli (bacteria), and small numbers of erythrocytes also noted in these areas. Bronchioles frequently contain similar inflammatory debris and bacteria, and focally, necrosis of bronchiolar epithelia is detected. Widespread hyperemia with small number of medium-caliber blood vessels variously exhibiting fibrinoid necrosis and leucocytoclastic vasculitis. Occasional thrombi noted (not visible in all sections).
Contributor’s Morphologic Diagnoses:
Lung: necrotizing bronchopneumonia, acute, severe with myriad bacteria (identified as Bordetella bronchiseptica on culture).
Contributor’s Comment: Bordetella bronchiseptica is a gram-negative coccobacillus commonly carried in the nasopharynx of healthy cats. In a survey of respiratory pathogens in multi-cat (³5 cats) households, B. bronchiseptica was detected by PCR in 5% of cats from households with disease, and in 1.3% of cats without.6 Considered a somewhat uncommon pathogen in this species, infection typically manifests when pulmonary defenses are impaired by feline calicivirus or herpes virus infections, or by stressful environmental conditions (while occasional structures possibly suspicious of basophilic intra-nuclear inclusions were observed in bronchiolar gland epithelium in this case, these were considered equivocal: immunohistochemistry was not performed).2,4,10 The clinical consequences on infection can vary dramatically: from mild pyrexia, coughing and sneezing to severe pneumonia and death as seen in this case.
Details of the pathogenesis of infection in the cat are inferred from studies in other species. Bacterial virulence is regulated by the Bordetella virulence gene (bvg) operon, which orchestrates the expression of the adhesin filamentous haemagglutinin (FHA), pertactin, and the fimbriae that facilitate adherence to the ciliated epithelium, as well as to macrophages and neutrophils within the lungs.2,6 Following attachment the pathogen secretes the RTX adenylate cyclase toxin (hemolysin) that results in impaired leucocyte phagocytosis and oxidative burst, and may induce their apoptosis. A lipooligosaccharide with endotoxin activity and a soluble peptidoglycan-derived tracheal cytotoxin are also produced that induce ciliostasis and the apoptosis of ciliated epithelial cells.2,6
Bacterial culture (as in this case) and PCR are used to conform the diagnosis, although both lack sensitivity.6,9 In regions where vaccines against B. bronchiseptica are available, it is recommended that cats should not be routinely vaccinated, given the typically mild disease that occurs. However, vaccination is encouraged in units such a shelters where populations are in continuous turnover, and thus at increased risk of infectious disease.6 Organisms are shed in oral and nasal secretions of infected cats and infected dogs are considered an infection risk for cats.5,6 Rare zoonosis, typically in immunocompromised individuals, have been reported.8,11
Mycoplasma felis can colonize the upper respiratory tract of cats, but evidence suggests it is not a significant primary pathogen. Molecular detection techniques have found Mycoplasma spp in the lower respiratory tract of 15.4% of cats with respiratory disease, with M. felis, M. gateae and M. feliminutum the species identified. However, the pathogenic significance of their presence remains unclear.7 It is likely that M. felis contributed to the severity of the pneumonia in this case through opportunist secondary involvement.
This case represented a sudden and highly traumatic loss for the owner concerned as they witnessed the rapid clinical deterioration and death of their prize-winning animal over a period of 36 hrs. We may speculate that contact with other cats at the cat show provided a source of the infection, and how possibly the stress of transport or attendance at this event could have precipitated disease. In any event, the rapidity of disease progression suggests the strain of B. bronchiseptica involved was particularly virulent.
Contributing Institution:
Room 012, Veterinary Sciences Centre, School of Veterinary Medicine, University College Dublin, Belfield, Dublin 2, Ireland
http://www.ucd.ie/vetmed/
JPC Diagnosis: Lung: Bronchopneumonia, fibrinosuppurative and necrotizing, multifocal to coalescing, severe, with numerous bacterial colonies.
JPC Comment: The contributor has provided an excellent review of Bordetella bronchiseptica in this case, as well as the pathogenesis of the disease across species lines. Bordetella bronchiseptica is an important, if sporadic, pathogen of the respiratory system in a wide range of mammalian species. Previous WSC cases include Bordetella bronchopneumonia infection in a chinchilla (WSC 2014-2015, Conf 18, Case 4), an African green monkey (WSC 2011-2012, Conf 22, Case 3), squirrel monkeys (WSC 2007-2008, Conf 9, Case 3, pigs (WSC 1996-1997, Conf 20, Case 2), a rabbit (WSC 1995-1996), among others.
There are three species of Bordetella of veterinary importance, including B. bronchiseptica (whose dermonecrotic toxin also causes atrophic rhinitis in pigs and rabbits), B. hinzii which causes limited respiratory disease in turkey poults and rare septicemia in humans, and B. pertussus a potent pathogen in humans which may cause lower respiratory disease in lambs.
Well known for its involvement in non-fatal tracheobronchitis (aka “kennel cough”), a recent publication9 has detailed Bordetella’s role in fatal pneumonia as exemplified in this case. B. bronchiseptica has been identified in between 12 and 78% of dogs with lower respiratory tract infections, and 5-13% of cats.9 At a major university veterinary school, a retrospective study identified B. bronchiseptica via immunohistochemistry and/or bacterial culture in 8 of 36 of canine and 14 of 31 feline cases of fatal bronchopneumonia. Upon close review, bacteria could be visualized among the cilia in 4 of 36 canine cases and 5 of 31 feline cases.9 Close review of columnar epithelium in this case also disclosed the presence of cilia-associated bacteria; it was seen only on intact cells, as necrotic or attenuated epithelium has probably already lost its cilia. This may be an important finding in such cases, as the lesions associated with the bronchopneumonia in these cases, in the authors’ opinion, were not specific for any particular pathogen.
Bordetella bronchiseptica can be an opportunistic pathogen in humans as well, particularly immunosuppressed individuals and cystic fibrosis patients. B. bronchiseptica and B. pertussis, (the causative agent of whooping cough) exhibit little genetic variation,1 with B. pertussis being a more recent derived, human adapted bacterium derived as a consequence of gene deletions and loss of genetic regulatory functions. Both organisms possess the Bordetella virulence gene as discussed above. In a large children’s CF center, 7 patients had multiple repeated isolates of B. bronchiseptica from their airways. All patients had documented exposure to pets or lived on a farm or an operating kennel, resulting in an overall infection of as many as 12% of children in the center at any time.1
One of the issues associated with persistence of Bordetella infections in human patients leading to vaccine failures and re-emergence of disease has been recently elucidated – the ability for this bacterium to produce biofilms.3 This is yet another feature of the BvgAS gene, which regulates the expression of genes encoding surface membrane, secreted, and regulatory proteins from host cells, as well as filamentous hemagglutinin, pertactin, fimbriae and various toxins which contribute to the extracellular matrix comprising the Bordetella biofilm.3
The moderator reviewed the general patterns of pneumonia in the lung across animal species. In this particular case, attendees were split on whether the appropriate morphologic diagnosis would be bronchopneumonia (based on the traditional pathogenesis of this aerogenous bacteria and the obvious necrosis within airways) or bronchointerstitial, based on the widespread necrosis of alveolar septa, pulmonary vessels, and exudative alveolitis in much of the section
References:
- Brady C, Ackerman P, Johnson M, McNamara J. Bordetella bronchiseptica in a pediatric cystic fibrosis center. J Cystic Fibrosis, 2014 13:43-48
- Caswell JL, Williams KJ: The Respiratory System. In: Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, ed. Maxie MG, 5th, vol. 2, Philadelphia, PA: Elsevier; 2007: 216-219.
- Cattelan N, Dubey P, Arnal L, Yantorno OM, Deora R. FEMS Pathog Dis 2016; doi 10.1093/femspd/ftv108.
- Coutts AJ, Dawson S, Binns S, Hart CA, Gaskell CJ,Gaskell RM. Studies on natural transmission of Bordetella bronchiseptica in cats. Vet Microbiol 48:19–27, 1996.
- Dawson S, Gaskell CJ, McCracken CM, Gaskell RM, Hart CA, Jones D. Bordetella bronchiseptica infection in cats following contact with infected dogs. Vet Rec 146: 46–48, 2000.
- Egberink H, Addie D, Belák S, Boucraut-Baralon C, Frymus T, Gruffydd-Jones T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Grazia Pennisi M, Radford AD, Thiry E, Truyen U, Horzinek MC. Bordetella Bronchiseptica Infection in Cats: ABCD guidelines on prevention and management. J Fel Med Surg 11:610-614, 2009.
- Reed N, Simpson K, Ayling R, Nicholas R, Gunn-Moore D. Mycoplasma species in cats with lower airway disease: improved detection and species identification using a polymerase chain reaction assay. J Fel Med Surg 14: 833–840, 2012.
- Register KB, Sukumar N, Palavecino EL, Rubin BK, Deora R. Bordetella bronchiseptica in a paediatric cystic fibrosis patient: Possible transmission from a household cat. Zoonoses Public Hlth 59: 246–250, 2012.
- Taha-Abdelaziz K, Bassel LL, Harness ML, Clark ME, Register KB, Caswell JL. Cilia-associated bacteria in fatal Brodetella bronchiseptica pneumonia of dogs and cats. J Vet Diagn Investig 2016; 28(4)369-376.
- Welsh RD. Bordetella bronchiseptica infections in cats. J Am Anim Hosp Assoc 32: 153–158, 1996.
- Woolfrey BF, Moody JA. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev 4: 243–255, 1991.