WSC 2022-2023
Conference 19
Case II:
Signalment:
9-year-old, castrated male, Netherland dwarf rabbit, Oryctolagus cuniculus domesticus
History:
This rabbit presented to the rDVM with chronic uveitis which was poorly responsive to treatment with oral and topical non-steroidal anti-inflammatory drugs and antibiotics. The rabbit otherwise appeared to be in good health. The owners opted for enucleation and submission for of the eye for histopathology.
Gross Pathology:
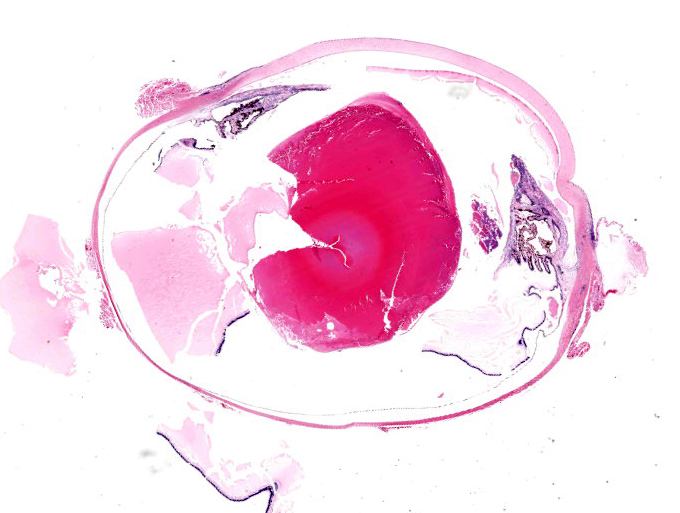
The submitted eye was grossly unremarkable.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
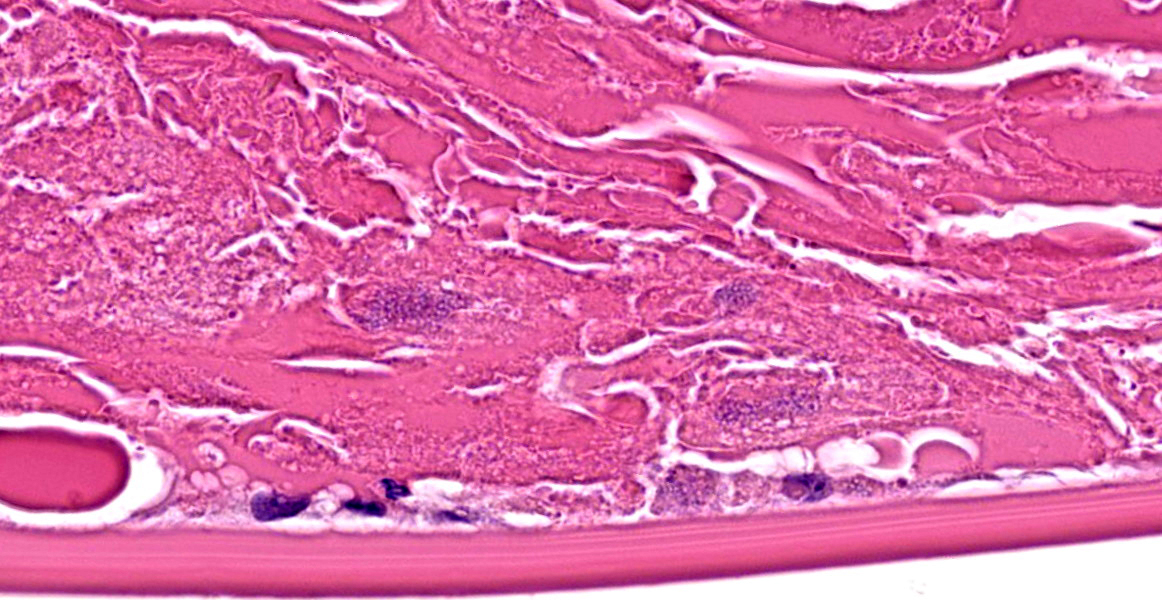
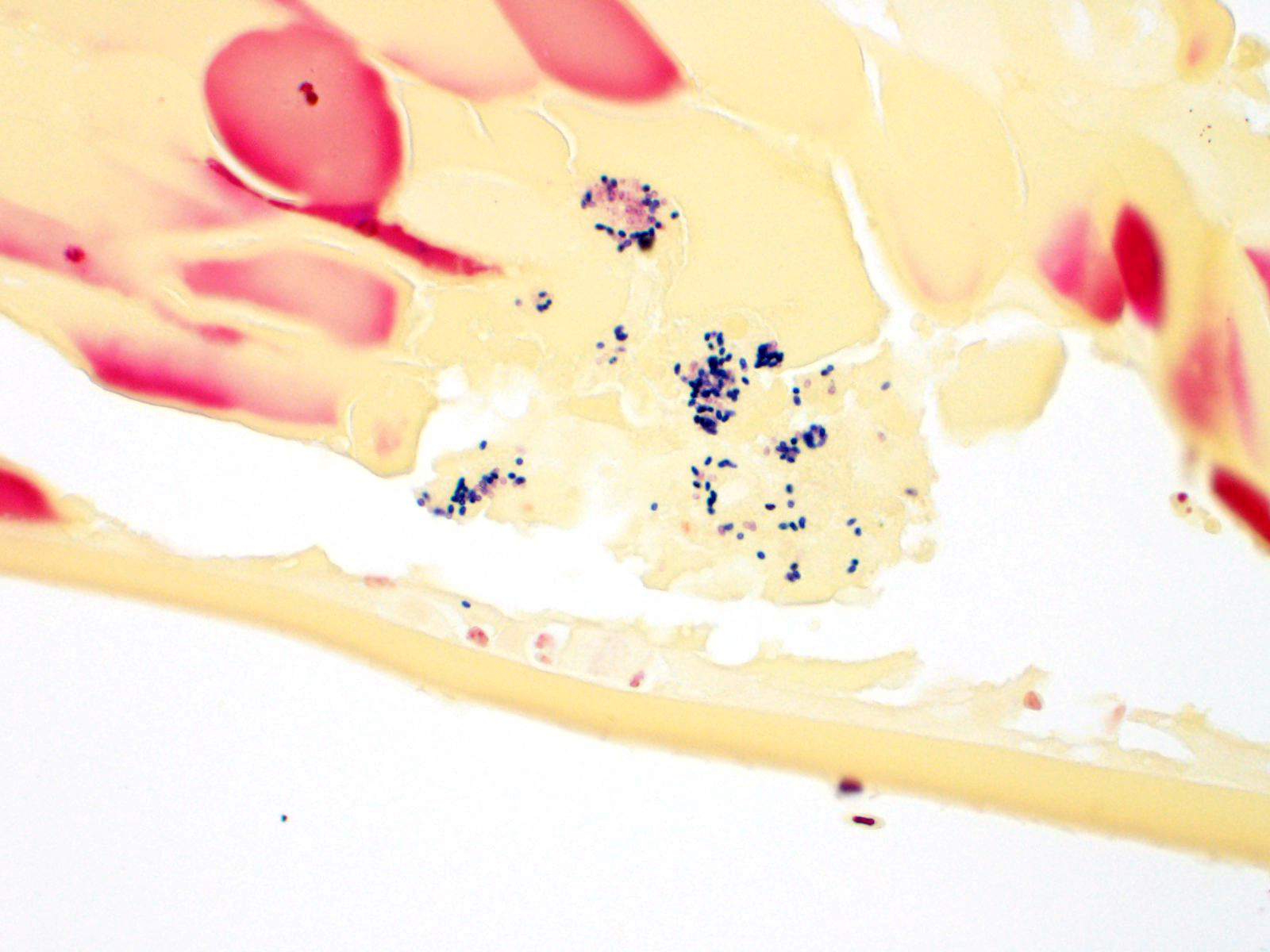
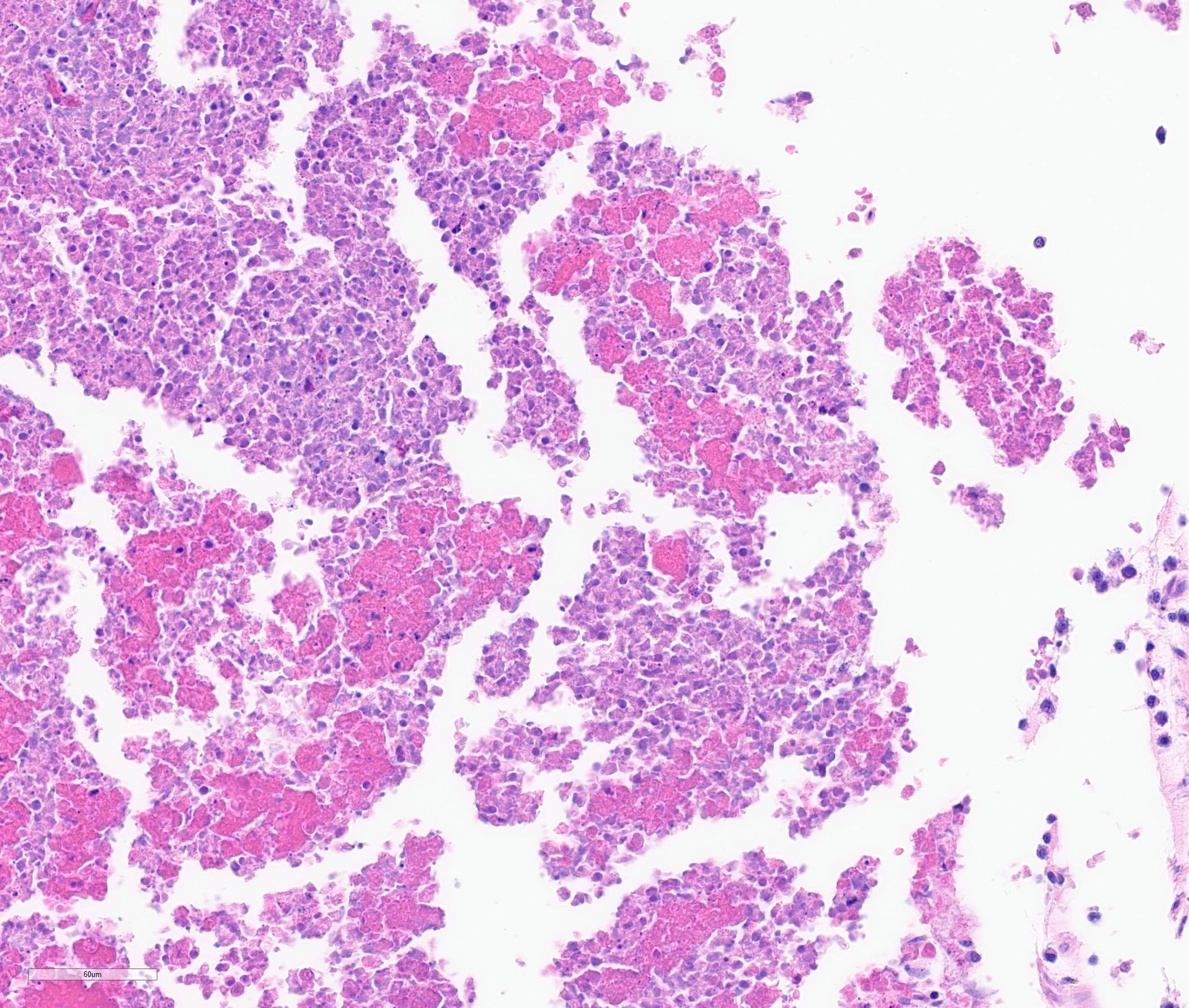
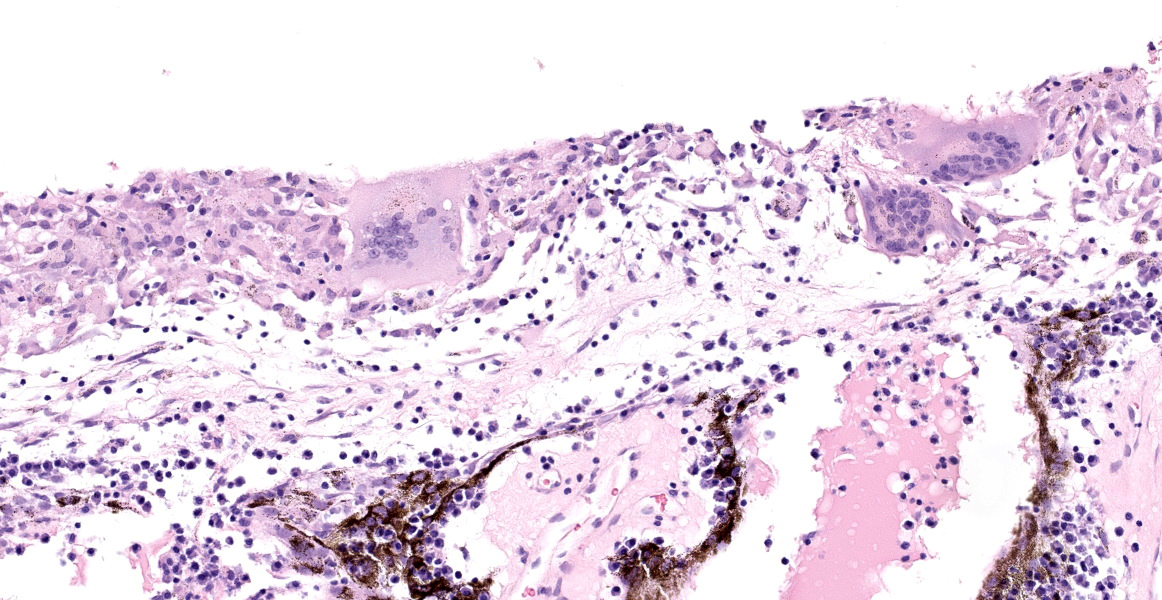
The iris contains moderate diffuse infiltrates of plasma cells accompanied by small numbers of lymphocytes and few scattered heterophils. Occasional strands of fine fibrous stroma associated with moderate aggregates of epithelioid macrophages, fewer multinucleated giant cells, occasional heterophils, proteinaceous debris and a few erythrocytes are noted in the anterior and posterior chambers. Thin strands of fibrous tissue rarely extend from the posterior iris near the pupillary margin. There are large areas where subcapsular lens fibers appear fragmented, rounded and are replaced by amorphous pink debris. Small amounts of basophilic, fine mineral, as well as numerous, 1-2 micron long, ovoid, gram positive, spores, are present beneath the lens capsule and within lens epithelial cells. Lens epithelial cells are often vacuolated and rarely form small piled up aggregates. In some areas, subcapsular lens fibers are lost and replaced by sparse cell debris.
Contributor’s Morphologic Diagnoses:
Eye: Moderate, chronic, plasmacytic and granulomatous, anterior uveitis with cataracts and numerous intralenticular, gram positive, microsporidial spores (consistent with Encephalitozoon cuniculi)
Contributor’s Comment:
The clinical history and microscopic appearance of the ocular lesions in this rabbit are most compatible with cataract formation and phacoclastic uveitis due to the ocular form of Encephalitozoon cuniculi infection.
Encephalitozoon cuniculi is a microsporidial pathogen with worldwide distribution. Although previously phylogenetically classified as a protozoa, it is currently considered a fungus.5,6 There are three major genotypes of E. cuniculi. Genotype 1 (or the rabbit strain) is generally associated with disease in pet and commercially raised rabbits. This organism has a direct life cycle. Horizontal transmission is most commonly through the ingestion of spores in urine-contaminated feed, or less commonly via inhalation. Vertical (or transplacental) transmission may also occur and is often hypothesized to be the cause of intra-ocular infections which facilitates access to, and colonization of lens fibers during fetal development.6 Typically, in the intestine, the spores primarily infect host cells through extrusion of their polar filament and injection of sporoplasm into a host cell. Phagocytosis by host cells has also been demonstrated.6 Initial target organs for infection via horizontal transmission are those with high blood flow such as the lungs, liver and kidney but many sites may be involved. Infection of the central nervous system (CNS) generally occurs later in the course of disease. At tissue sites, sporogony continues and eventually leads to rupture of host cells and the release of spores which induces a localized lymphoplasmacytic to granulomatous inflammatory
response. Most immunocompetent animals have subclinical infections with foci of granulomatous inflammation most commonly present in the brain, kidneys, and eye.5,6 Spores are shed intermittently in the urine of infected rabbits. Clinical disease in rabbits is most often seen in older rabbits as immunocompetency wanes with age or following stressors that may have a negative impact on the host immune response (ie. changes in environment, other illnesses, medications, etc). In rabbits, the most common manifestations of E. cuniculi infection are clinical signs of central nervous system disease, uveitis, and chronic renal disease.
Rabbits with encephalitozoonosis most commonly present with a variety of neurologic clinical signs resulting from foci of lymphoplasmacytic to granulomatous meningoencephalitis. Many affected rabbits develop central vestibular disease which presents as head tilt, ataxia, nystagmus, and/or circling movements. More severe clinical signs may include rotation along the body length axis (ie. rolling) or lateral recumbency which are generally associated with a poorer prognosis.5 The main clinical differential diagnosis in these rabbits is peripheral vestibular disease due to otitis interna. Other neurologic signs that may be associated with encephalitozoonosis include seizures, paresis, altered mentation and behavioural changes.
Ocular lesions are most frequently reported in young rabbits with no other apparent clinical signs and lesions are most often unilateral. However, bilateral lesions have been documented in rabbits.5 In some cases (likely largely older individuals), other clinical signs (especially neurologic signs) may also be noted.8 In the eye, spores infect the lens epithelial cells and when these cells rupture it results in lens fiber degeneration, cataract formation and eventually phacoclastic uveitis.
Rabbits with significant renal disease resulting from chronic encephalitozoonosis often have slightly pale, pitted kidneys that microscopically exhibit lymphoplasmacytic to granulomatous, tubulointerstitial nephritis with fibrosis. These rabbits generally present with non-specific clinical signs of polyuria, polydipsia, weight loss, dehydration, and anorexia.
In tissue sections, within foci of inflammation, microsporidial organisms are Gram positive which is a useful means of highlighting spores. Immunohistochemistry (IHC) and PCR testing of tissues, especially the eye contents, has been used to try to confirm a diagnosis of encephalitozoonosis. Unfortunately, PCR and IHC testing for diagnostic specimens is not easily accessible. Antemortem diagnosis of E. cuniculi remains a challenge. Encephalitozoon cuniculi is considered zoonotic, especially in immunocompromised people who are at an increased risk of acquiring infection.
Contributing Institution:
Atlantic Veterinary College, University of Prince Edward Island
JPC Diagnosis:
Eye, globe: Uveitis, phacoclastic, granulomatous, diffuse, moderate, with cataractous change and intralenticular microsporidial spores.
JPC Comment:
This is a classic case of phacoclastic uveitis caused by E. cuniculi. These microsporidian parasites have a characteristic coiled polar filament visible on electron microscopy as parallel strips winding around the nucleus, or if viewed in cross section, as regularly spaced circles along the periphery of the organism.3 As the contributor mentioned, there are several genotypes of E. cuniculi, with genotypes II, III, and IV representing murine, canine, and human strains, respectively, though the genotypes are not exclusive to a single host species.3E. cuniculi can infect a variety of species, including guinea pigs, mice, rats, hamsters, primates, foxes, birds, dogs, and humans.1,3 Ocular E. cuniculi infections have been sporadically documented in other species; for instance, cataracts have been reported in a snow leopard, dogs, mink, and blue foxes.
Two reports have described E. cuniculi cataracts in domestic cats in Austria and California. In a 2011 report by Benz et al, positive E. cuniculi titers were found in 11 cats (total of 19 eyes) with uveitis and cataracts in Austria.2 In 18 of the eyes, E. cuniculi was identified on PCR of lens material.2 In 11 of the eyes, histopathology with acid-fast trichrome of the anterior lens capsule revealed E. cuniculi spores in the lens epithelial cells.2 The authors concluded that a positive E. cuniculi titer in conjunction with cataracts is strongly indicative of active infection in cats.2 Similarly, E. cuniculi infection was diagnosed in three cats with immature cataracts and uveitis in California based on lens histopathology (hematoxylin and eosin or Ziehl-Neelsen) or PCR, and serologic testing was positive in the two tested cats.7 These reports illustrate that E. cuniculi can induce similar ocular lesions in cats as in rabbits.
Control of the infection appears to be linked to the Th1 immune response and IFN-gamma production.3 Studies have shown an initial CD4+ T-cell proliferation in the spleen followed by CD8+ T-cell proliferation, and high levels of IFN-gamma can be detected in the spleen, mesenteric lymph nodes, and Peyer’s patches.3 Humoral immunity does not appear to offer sufficient protection against infection but may aid in diagnosis as serologic testing is one of the few antemortem tests available.3 Antibody titers initially rise by 4 weeks post infection and are high by 9 weeks.1 Infected animals have an initial spike in IgM followed in a few weeks by an IgG spike.3 Since a single positive titer cannot differentiate active infection from previous exposure, serial sampling to evaluate the titer differences is crucial. Perhaps the most helpful test is a negative titer: while a single negative test may happen in acute infections, paired negative titers spaced three weeks apart can rule out E. cuniculi infection.3
Spontaneous and congenital cataracts also occur in domestic rabbits and should be considered as a differential diagnosis for E. cuniculi-induced cataracts.1 The incidence of cataracts in New Zealand white rabbits is suggestive of an inherited autosomal recessive defect in this breed.1 As the contributor mentions, intra-ocular infection by E. cuniculi in rabbits is speculated to be due to in utero infection of the developing lens; however, a recent study in specific-pathogen free rabbits demonstrated lens after experimental oral infection with E. cuniculi, indicating other routes of infection may be possible.4
References:
- Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. 4th Ames, IO: John Wiley & Sons, Inc. 2016; 79, 146, 186, 234, 293-295, 318.
- Benz P, Maas G, Csokai J, et a. Detection of Encephalitozoon cuniculi in the feline cataractous lens. Vet Ophthalm. 2011; 14 (Suppl 1) 37-47.
- Dobosi AA, Bel LV, Pasitu AI, Pusta DL. A Reivew of Encephalitozoon cuniculi in Domestic Rabbits (Oryctolagus cuniculus)-Biology, Clinical Signs, Diagnostic Techniques, and Prevention. Pathogens. 2022; 11: 1486-1500.
- Jeklova E, Leva L, Kummer V, Jekl V, Faldyna. Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits. Animals (Basel). 2019; 9(11): 988-995.
- Kunzel F, Fisher PG. Clinical signs, diagnosis, and treatment of Encephalitozoon culiculi infection in rabbits. Vet Clin Exot Anim. 2018; 21:69-82.
- Latney LV, Bradley CW, and Wyre NR. Encephalitozoon cuniculi in pet rabbits: diagnosis and optimal management. Vet Med Res. 2014; 5:169-180.
- Lin J, Nell B, Horikawa T, Zarfoss. Feline intraleunticular Encephalitozoon cuniculi: three cases from California. JFMS Open Rep. 2022; 8(2): 1-9.
- Morsy EA, Salem HM, Khattab MS, Hamza DA, and Abuowarda MM. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet Scand. 2020; 62:11