Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 3
4 September, 2019
Dr. Corrie Brown, DVM, PhD, Diplomate ACVP
Josiah
Meigs Distinguished Teaching Professor
University Professor
Department
of Pathology
University of Georgia College of Veterinary Medicine
2200 College Station Road Athens, GA, 30602
CASE IV: P17-969 (JPC 4135861).
Signalment: 7 months old, female, Holstein, Bos taurus, bovine.
History: The animal was experimentally infected with 10,000 ID50 foot-and-mouth disease virus (FMDV A IRN/22/2015) into the tongue. After 24 hours, the animal was euthanized and submitted for necropsy.
Gross Pathology:
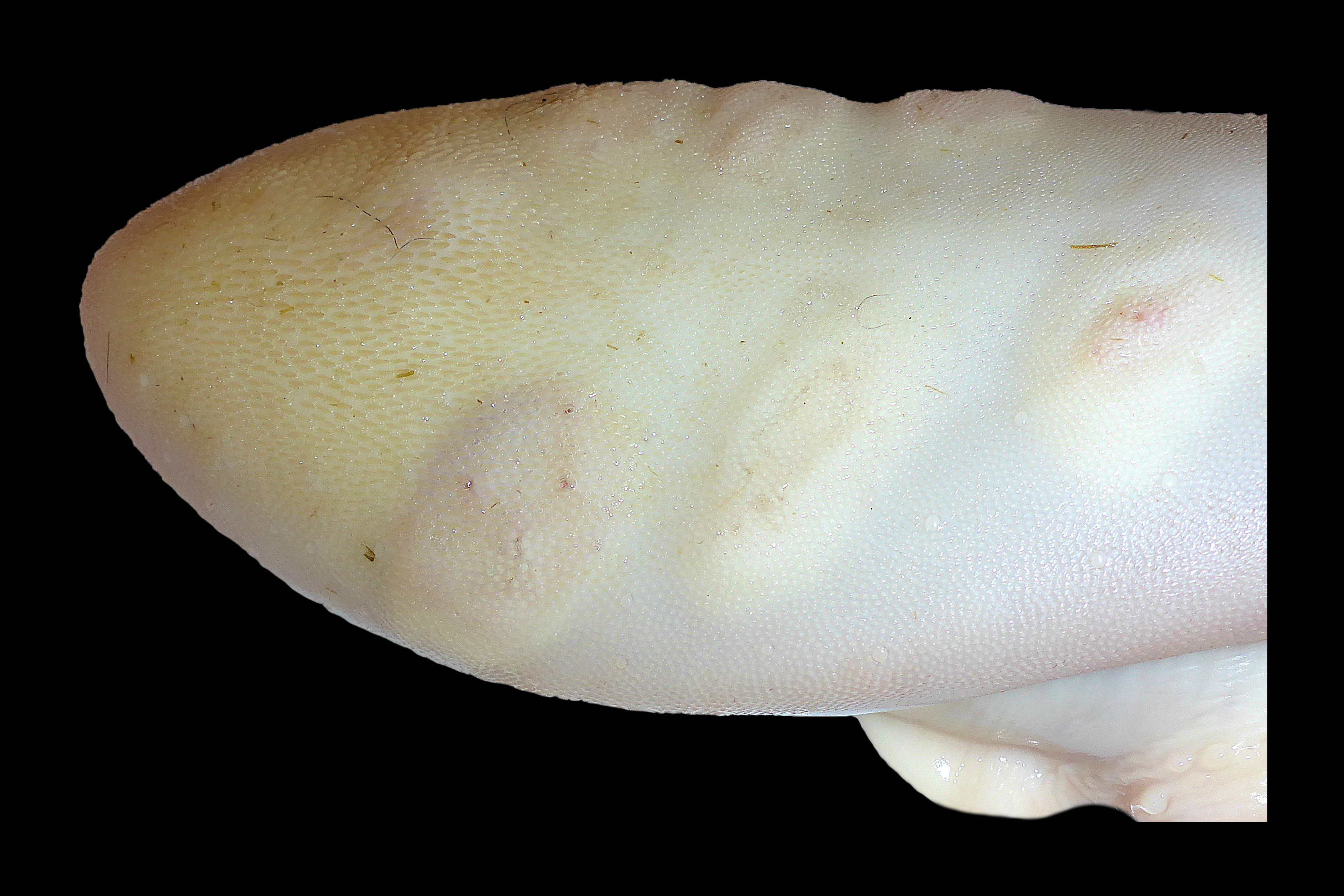
Tongue: Multiple oval to round vesicles (aphthae), up to 3 cm diameter were multifocally present at the dorsum of the tongue.
Hoof: Multiple vesicles and pustules were also found on the left hoof within the interdigital space and bulb of the heel.
Laboratory results: None.
Microscopic Description:
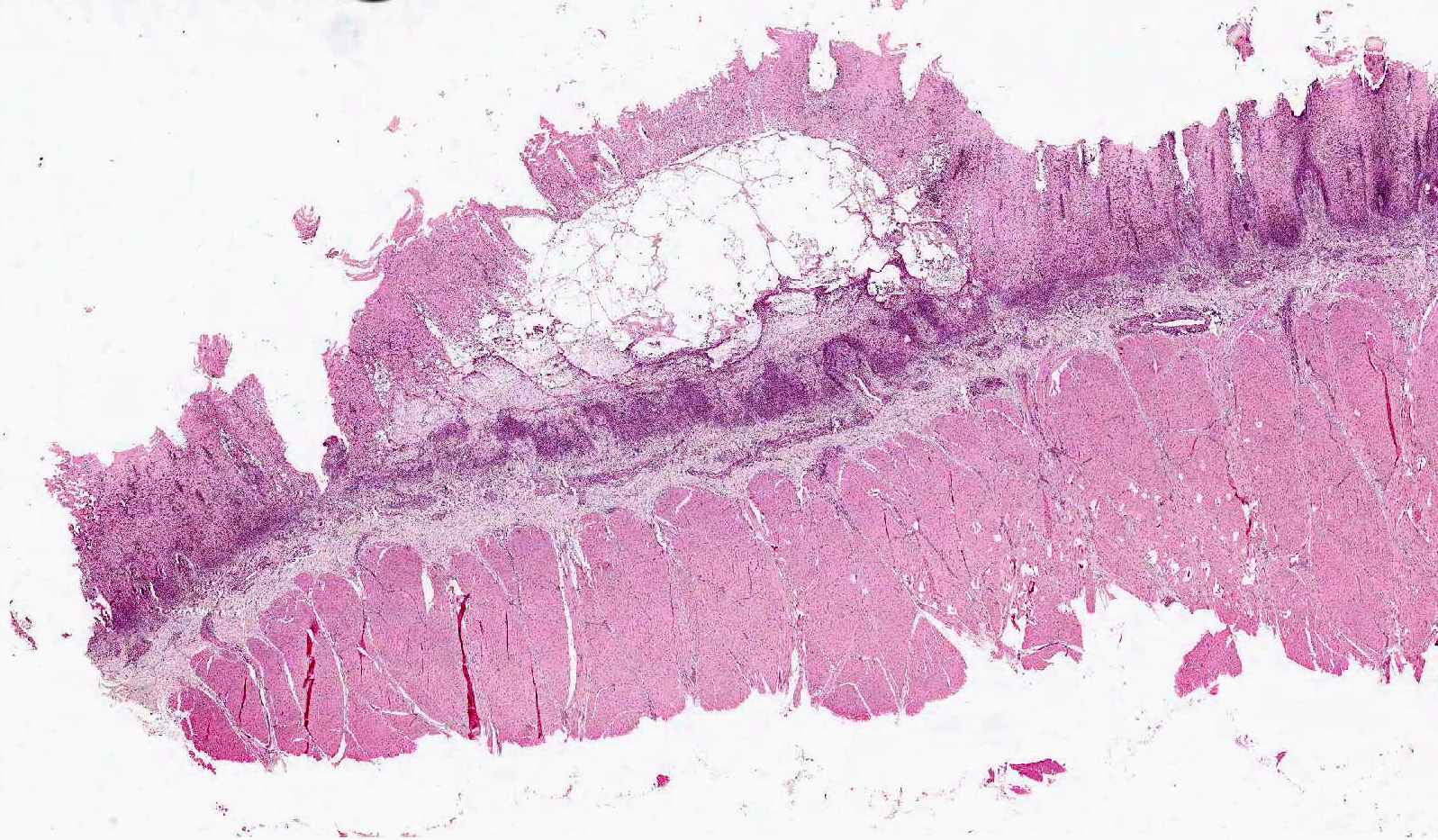
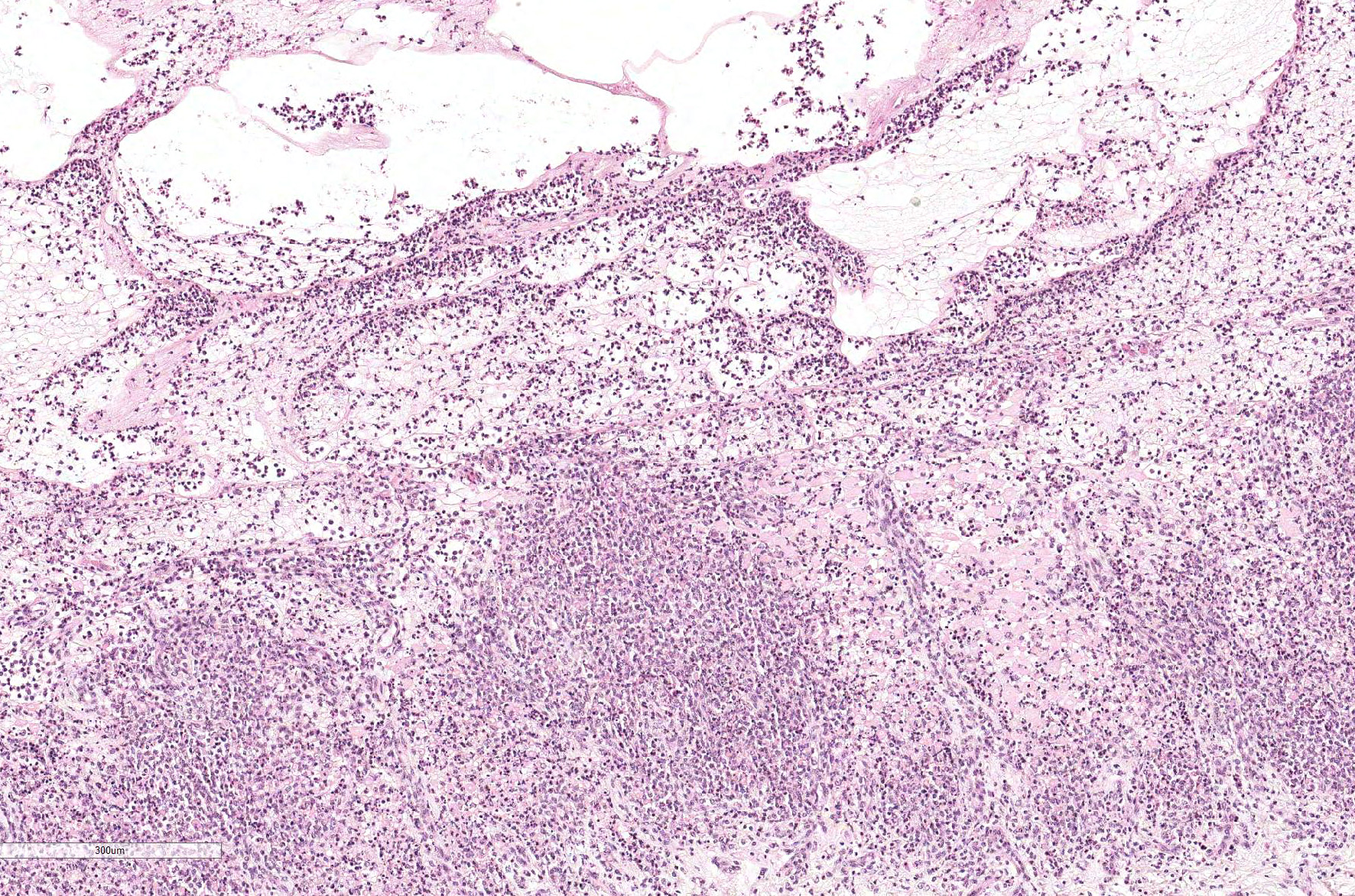
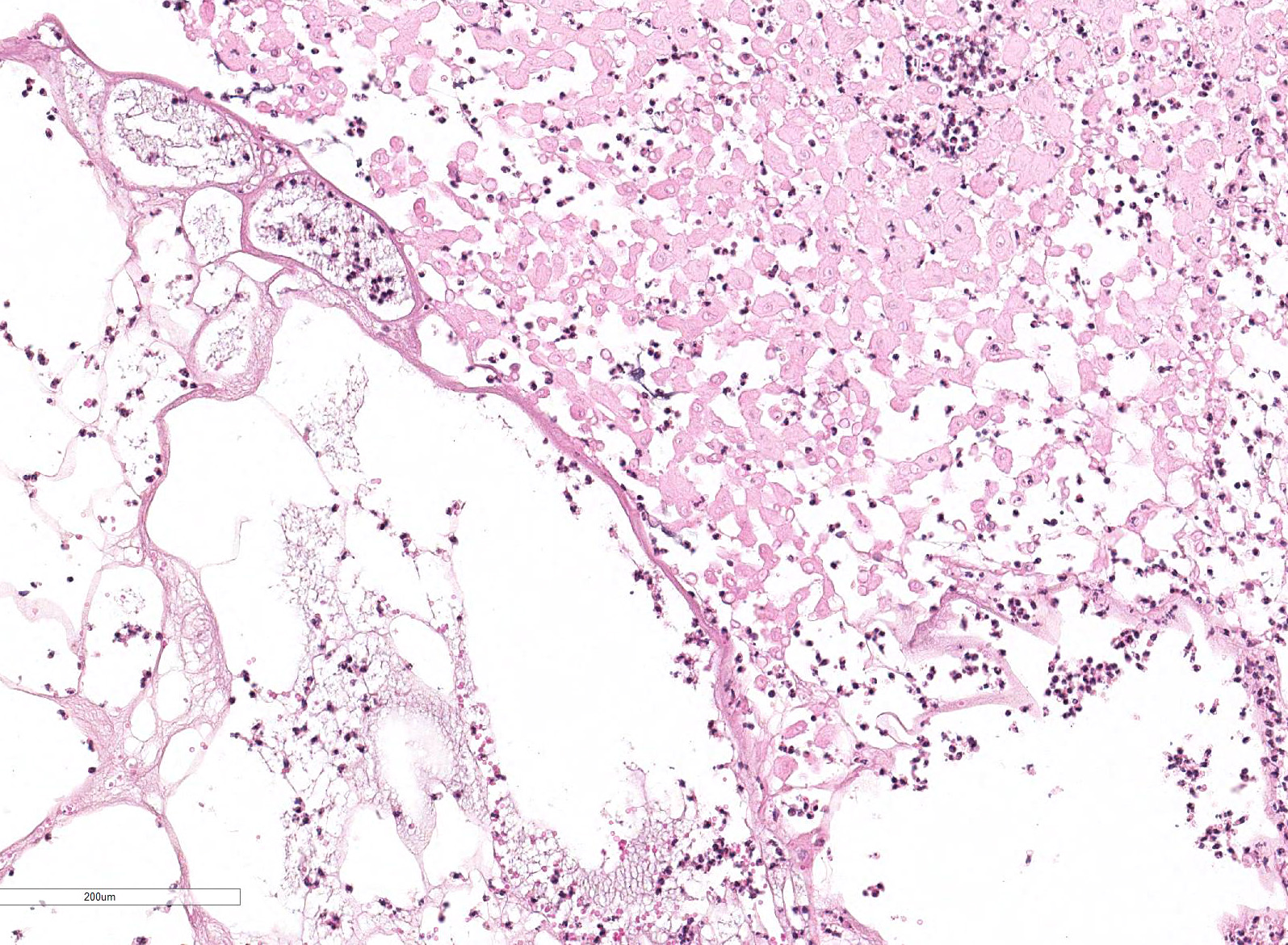
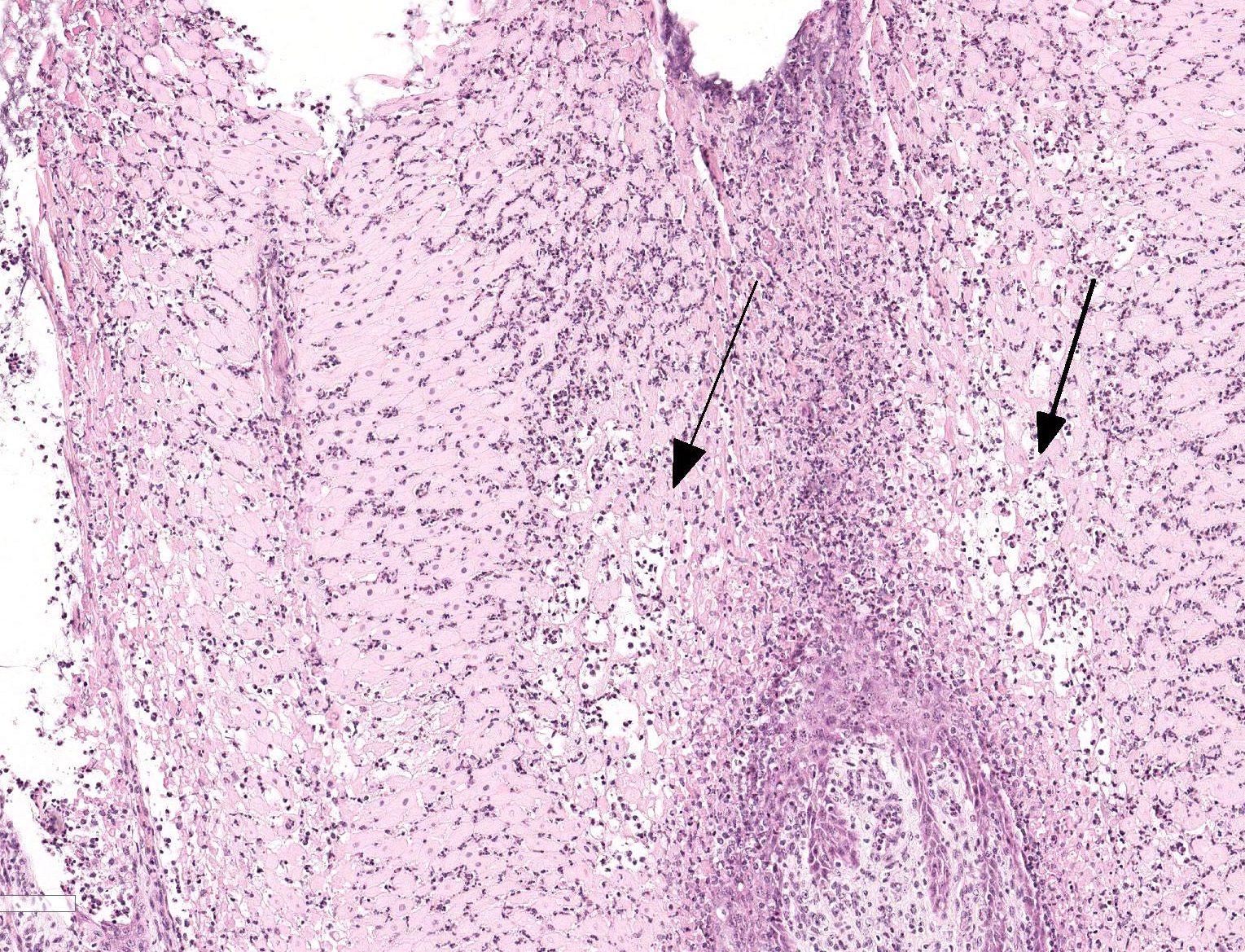
Tongue. Expanding within the lingual mucosa, there is a 6.5 mm large, well-demarcated vesicle (vesiculopustule) which contains variable numbers of viable and degenerate neutrophils, erythrocytes, fibrillar beaded eosinophilic material (fibrin) and a low amount of proteinaceous fluid. The adjacent epithelial cells are polygonal to round, swollen with hypereosinophilic cytoplasm, basophilic karrhyorectic and pyknotic nuclei (lytic necrosis), and are separated by intercellular edema (spongiosis). Diffusely, the epithelium is infiltrated by a large number of neutrophils, focally obscuring the basal epithelial layer. Predominantly, perivascular, moderate numbers of neutrophils and few macrophages are present within the submucosa and adjacent connective tissue. Endothelial cells are hypertrophic (endothelial activation).
Contributor Morphologic Diagnosis:
Tongue. Glossitis, vesiculopustular, focal, suppurative, moderate, acute with intramucosal edema, Holstein (Bos taurus), bovine.
Contributor Comment: Foot-and-mouth disease (FMD) is a highly contagious, viral disease of cloven-hoofed animals. The etiologic agent is the FMD virus (FMDV), a picornavirus, of which there are seven known FMDV serotypes: O, A, C, Asia 1, SAT1, SAT2, and SAT34,9. Although FMD outbreaks are often of low mortality rates, morbidity is often high due to the characteristic vesicles within the mouth and on the feet, resulting in profound agricultural production and economic losses2,9. Furthermore, the high level of contagiousness of FMD and high tenacity of the FMDV pose a considerable challenge in controlling a disease outbreak.
In the United
Kingdom in 2001, an outbreak of FMD resulted in the mass culling of over 6
million cattle and sheep, costing nearly $12 billion.9 From
2010-2011, three outbreaks occurred in Japan and South Korea, with 3 million
pigs, and 100,000 cattle being culled.9,10 Throughout the FMD
endemic regions, outbreaks are frequently reported and ongoing, including (as
of June 17, 2019) 6,370 cases in Algeria, 968 in China, 386 in Malawi, 239 in
Morocco, 6,391 in Mozambique, 1,200 in Sierra Leone, 1,220 in Zambia, and 2,108
in Zimbabwe5. Globally, in the past 5 years (as of June 17, 2019), FMD
outbreaks resulted in 376,367 cases, 12,201 deaths, 314,063 culls, with an
estimated 3,650,220 animals identified as susceptible5.
Although FMD constitutes a major global health threat, research into understanding the fundamental mechanisms of disease pathogenesis remains limited due to, in part, the necessary biosecurity and infrastructure to work with FMDV, as well as the diversity of viral serotypes and strains2. In general, FMD is designated into three stages: pre-viremia, viremia, and post-viremia2. The definitive location of viral entry remains to be elucidated, although the pharynx and larynx appear to be an important anatomical site.2,9 Furthermore, although infected animals develop a profound viremia 1-2 days before the onset of clinical signs, the source of the viremia also remains poorly understood.2,9 Vesicles often hold higher viral titers than the blood, however, viremia may occur prior to vesicle formation.2 Other tissues with viral titers include the skin (whether or not lesions are present), lungs, lymph nodes, and heart.2 After the clearance of viremia, approximately 50% of cattle may become persistent carriers, although to what degree the carriers pose a threat to naïve animals, also remains poorly understood.1,2,4
Clinically, FMD manifests as a fever 2-7 days post-infection, followed by the vesicle formation in the mouth and on the feet, which may rupture under mechanical stressors, thus forming large erosions7. Damage to the epithelium results in hypersalivation and lameness, ultimately leading to weight loss and a drop in milk production.9,10 Characteristic histologic lesions include vacuolar degeneration of epithelial cells, leading to hydrophobic cell swelling, cellular degeneration, lysis, and intraepithelial edema.9 Healing occurs first through fibrin exudation with neutrophilic granulocytes, to granulation tissue and re-epithelialization.9. In addition to classical lesions, FMDV also induces a fatal lymphocytic-histocytic myocarditis especially in young animals, characterized by small, grey-white foci within the myocardium of the left ventricle and septum.4,9
Important infectious differential diagnoses for ulcerative and erosive lesions include vesicular stomatitis (VS), vesicular exanthema in swine (VES), swine vesicular disease (SVD), bovine virus diarrhea (BVD), bluetongue virus (BTV), and malignant catarrhal fever (MCF).9 Non-infectious causes include burns, trauma, and toxicants (selenium toxicity, cantharidin (blister beetle), toxic plants (buttercup, St. Johnâs Wort, lantana, elderberry)), and photodermatitis.3,9 Differentiation of these diseases may be made through laboratory diagnostics, including detection of the pathogen (antigen ELISA, PCR, viral isolation), histopathology, or antibodies produced by the infected animal1,4,9 In many countries, prior to laboratory confirmation of disease, cases of vesicular lesions must be reported to official veterinarians in order to ensure prompt response and preparedness in the event of an FMD positive animal.
A primary objective in controlling FMD globally is immediate detection of disease, as well as controlling the movement of animals and animal products, and contacts with animals between countries..9,10 It is imperative that animal health practitioners are trained to not only identify early cases of FMD, but also practice high standards of biosecurity and workplace hygiene.9 Although FMDV is sensitive to changes in pH (below 6.0, above 9.0) and standard disinfectants (2% sodium hydroxide, 0.2% hydrochloric acid, 2% formalin, 0.5% citric acid), a challenge lies in controlling potentially contaminated materials, as all secretions and excretions of an infected animal are considered infectious.6 Furthermore, FMDV may survive in the soil for up to 28 days, in urine for 39 days, and fecal slurries for up to 6 months.6 In addition, cattle that become carriers may continue to harbor virus for up to 3.5 years1. Vaccinations are available, however, a vaccine is not protective across serotypes, and vaccination does not prevent animals from becoming carriers.2,9
Contributing Institution:
Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Department of Experimental Animal Facilities and Biorisk Management, Südufer 10, 17493 Greifswald â Insel Riems, Germany. https://www.fli.de
JPC Diagnosis: Tongue, mucosa and submucosa:
Glossitis, vesicular and neutrophilic, multifocal to coalescing, severe.
JPC Comment: The contributor has done an outstanding job describing this disease of global veterinary importance with particular regard to cattle. The apthovirus that causes foot and mouth disease affects a range of other species, some of which may contribute significantly to the potentiation and duration of an outbreak. Small ruminants may facilitate the spread of outbreaks as lesions are often inapparent, and these animals are easily transported. In countries in the virus is endemic, African buffalos and impala have been documented as reservoir hosts and well as being able to pass the virus on to domestic cattle.7
Much of the available research has been performed on cattle; however, swine are
a major component of the worldâs meat production as well, and the pathogenesis
of the disease is significantly different in this species. Swine are
considered amplifier hosts and have the ability to produced large amounts of
virus, which may be transmitted by aerosols to other farms. While the clinical
signs of FMD in swine are similar to those in cattle, the pathogenesis of the
disease in swine demonstrates considerable different.8 Pigs are
more susceptible to oral inoculation (through oropharyngeal tonsillar tissue)
than via aerosol exposure which suggests that physical separation of pigs may
be successful to decrease infection rates in swine, but not in cattle. During
clinical disease, the highest quantities of virus is produced within vesicles
in the oral cavity and feet; however, pigs exhale massive quantities of
infectious FMDV, likely with tonsillar tissue as the source.8 Convalescence
occurs within 1-2 weeks, although serious injuries to the feet may resulting in
secondary infection and persistent lameness. Pigs that clear the disease also
apparently completely clear the virus with 17 days; carrier’states apparently
do not occur in this species.8
The moderator showed the progression of vesicles from slides in her personal collection taken from experimentally infected animals and discussed the importance of the integrin receptor on target cells for invasion and dissemination of the FMD virus. Integrins are cell surface receptors which are distributed widely on the surface of cells of the stratum spinosum. This particular receptor results in the specific location of vesicles in the middle aspects of the lingual mucosa as seen in this case, as well as the species specificity of the virus, with cattle and pigs sharing a similar receptor, and horses not possessing this specific integrin, which makes them resistant to FMD.
References: 1. Alexandersen S, Zhang
Z, Donaldson AI. Aspects of the persistence of foot-and-mouth disease virus in
animals â the carrier problem. Microbes and Infection. 2002; 4:
1099-1110. 2. Arzt J, Juleff N,
Zhang Z, Rodriguez LL. The pathogenesis of foot-and-mouth disease I: Viral
pathways in cattle. Transboundary and Emerging Diseases. 2010; 58: 291-304. 3. Holliman A.
Differential diagnosis of diseases causing oral lesions in cattle. In
Practice. 2005; 27: 2-13. 4. Longjam N, Deb R,
Sarmah AK, Tayo T, Awachat VB, Saxena VK. A brief review on diagnosis of
foot-and-mouth disease of livestock: conventional to molecular tools. Veterinary
Medicine International. 2011. 5. OIE. World Animal
Health Information Database. Accessed 17 June 2019.
6. Owen JM. Disinfection
of farrowing pens. Rev. sci. tech. Off. Int. Epiz. 1995; 14(2): 381-391. 7.
Paton DJ, Gubbins S, King DP. Understanding the transmission
of foot-and-mouth disease virus at different scales. Curr Opin Virol
2018; 28:85-91. 8.
Stenfeldt C, Diaz-San Segundo F, de los Santos T, Rodriguez
LL, Arzt J. The pathogenesis of foot-and-mouth disease in pigs. Front Vet
Sci 2018; 3:41:10.3389/fvets.2016.00041. 9. Teifke JP, Breithaupt
A, Haas B. Foot-and-mouth disease and its main differential diagnoses. Veterinary
Practice of Large Animals. 2012; 40(G): 225-237. 10. Yoon H, Yoon SS, Wee
SH, Kim YJ, Kim B. Clinical manifestations of foot-and-mouth disease during the
2010/2011 epidemic in the Republic of Korea. Transboundary and Emerging
Diseases. 2012; 59: 517-525.