Signalment:
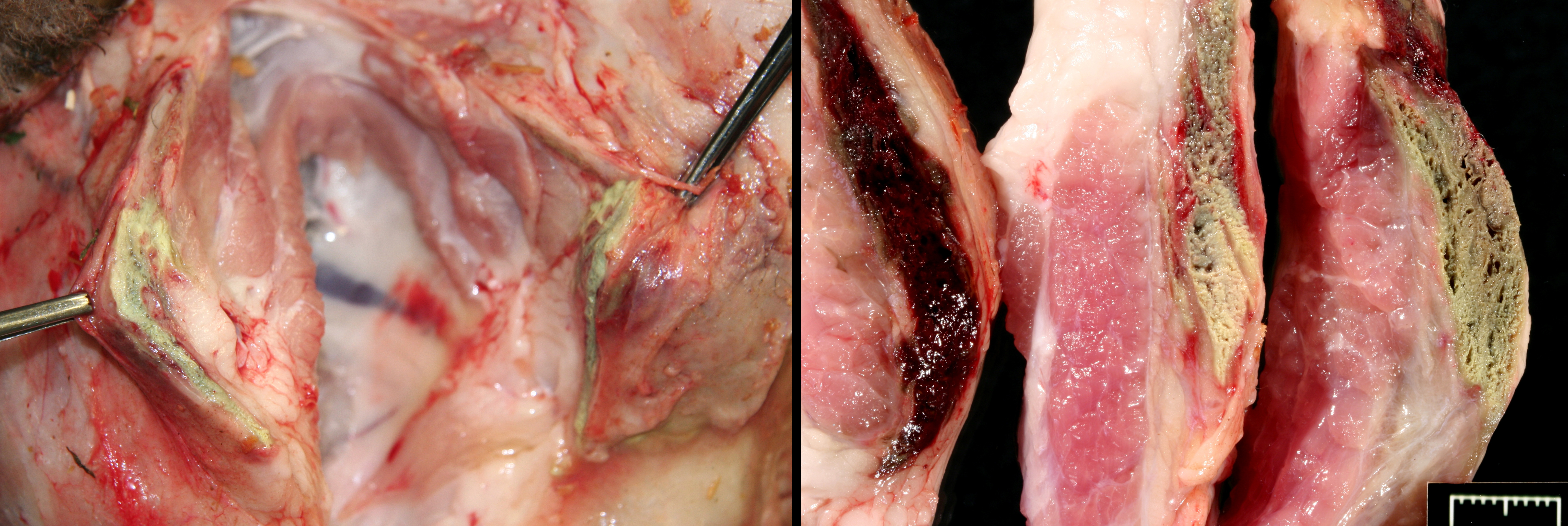
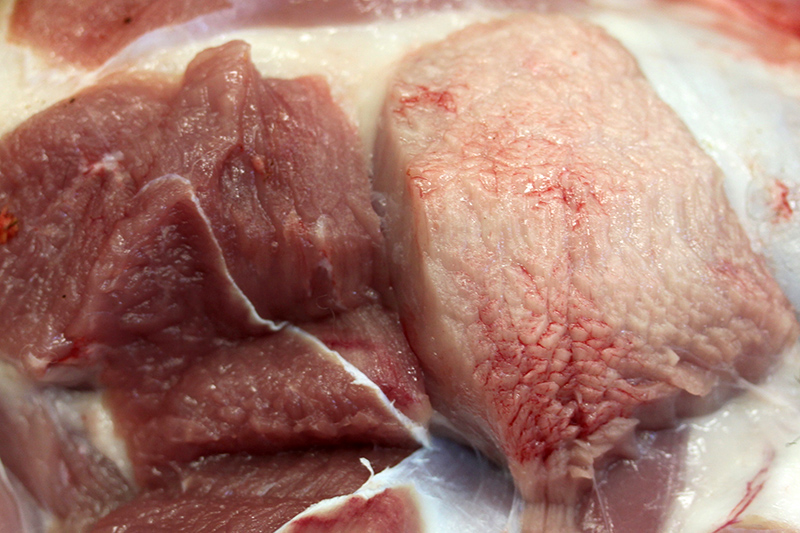
Gross Description:
Numerous Hemonchus and Trichostrongyles are in the abomasum.
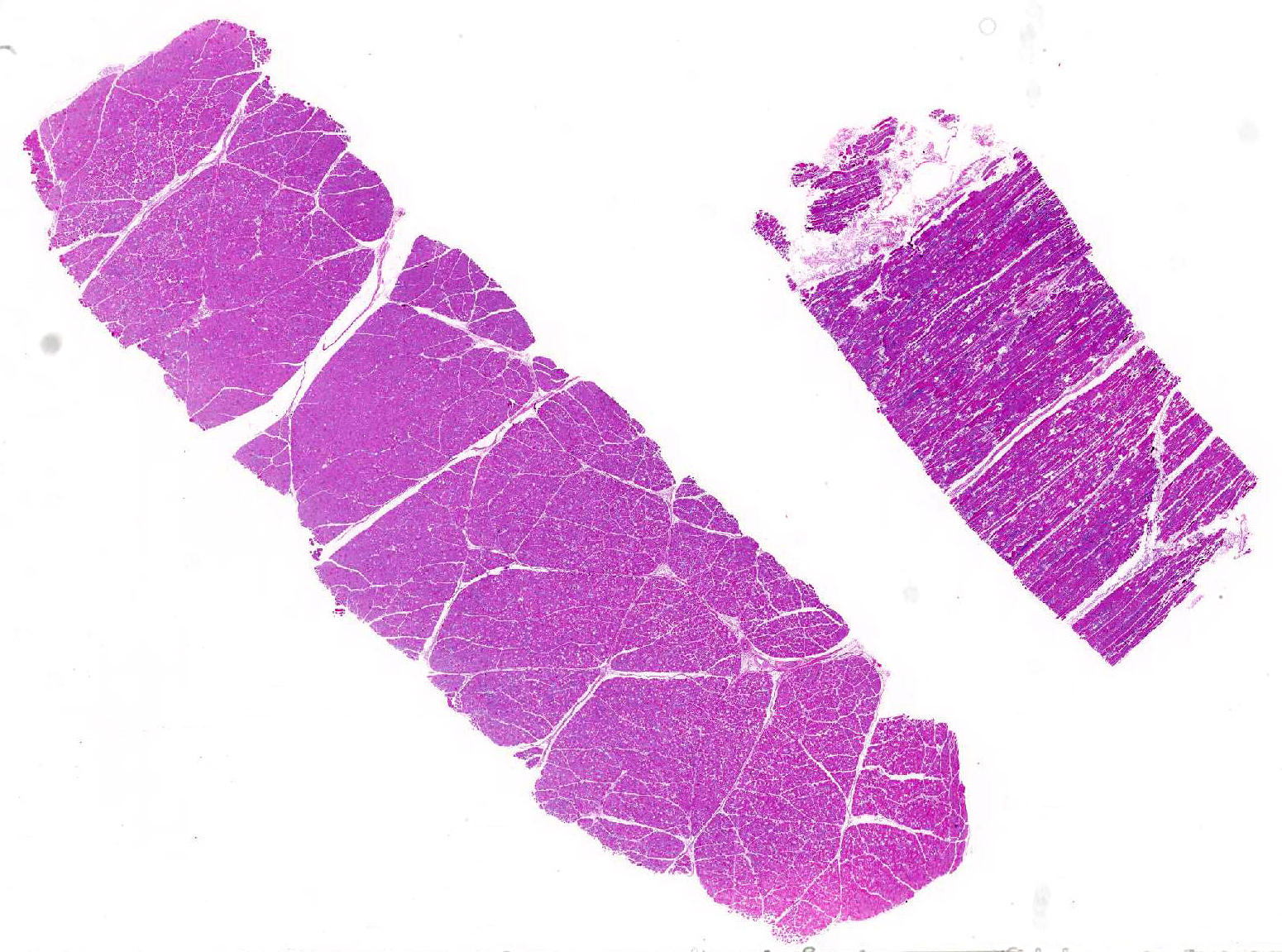
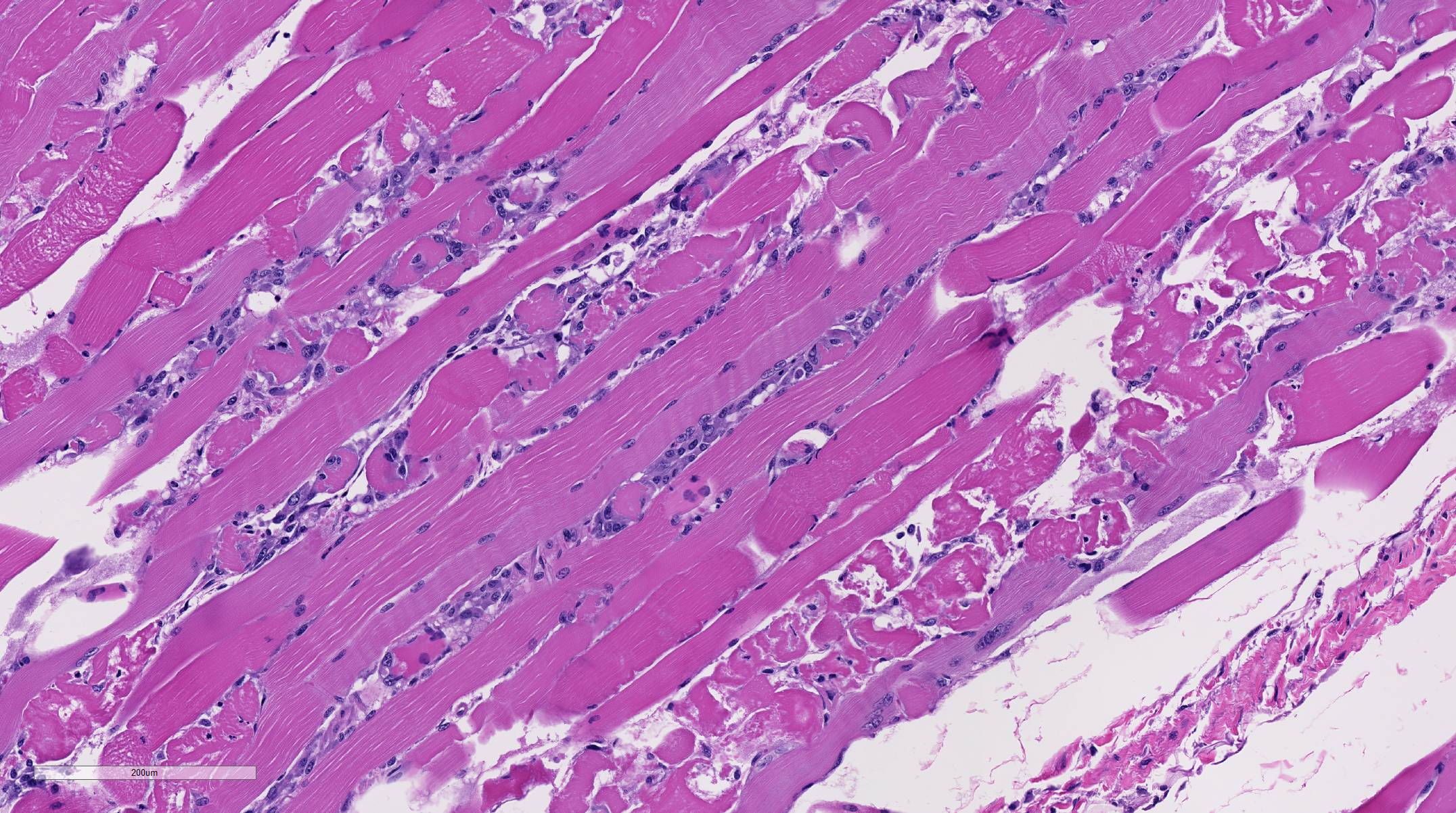
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
This breeder had been having a chronic, sporadic, neurologic problem in the flock of show sheep. It affected sheep taken off pastured and pushed nutritionally for the show season. Upon opening this ewe, it was obvious that a myopathy was the problem, and given the history, we analyzed the rumen content (negative for ionophores additives), and then, the feed from the bag used to fed these ewes was tested (toxic levels of lasalocid). The levels of lasalocid the feed were extremely high, and it is presumed a pocket of unmixed, lasalocid salt was included in the test sample. In some reports2 and in this contributors experience, once the ionophore is over a certain con-centration, these additives are unpalatable, and animals refuse the feed rather than consume myotoxic levels. Unfortunately, toxicity studies are based on bolus feedings.1,4
In spontaneous, ionophore (usually monensin) intoxications of sheep2,7,8,9,10 display an acute onset of signs of anorexia, dyspnea, muscle weakness, ataxia, a stiff gait and deaths follow feed changes. Firm or atrophic rear limb muscles are sometimes reported. A variety of autopsy lesions are reported including: cavitary effusions, pale-streaked hearts, diarrhea and pale-streaked skeletal muscles especially semi-membranosus and semitendinosus. While some acute gastrointestinal lesions are reported, consistent macroscopic and/or histologic lesions are in cardiac and skeletal muscles. In ovine monensin toxicity studies1,4, it was commented that acute myopathies were not visible in H&E-stained sections, but that lesions were demonstrable using electron microscopy. Early ultra-structure changes include mitochondrial swelling and myofibrillar disarray. Chronic histologic lesions include atrophy, fibrosis, and calcification. The severity of the present case is impressive, and the minimal cardiac lesion is unexplained. Is it possible that hypoxia from hemonchosis-exacerbated lesions?
Finally, many ionophore intoxications present as CNS disease. Although in light of the muscle lesions, the signs could be explained as muscular pain and weakness, we should remember that an ionophore neuropathy is observed in some toxicity trials.5 Our ewe had no lesions in the sciatic or femoral nerves, but some conduction problems may be occurring.
JPC Diagnosis:
Conference Comment:
Conference participants briefly reviewed the stages of skeletal muscle necrosis, re-generation, and repair. Myofibers are long and multinucleated and thus often undergo segmental necrosis rather than necrosis of the entire muscle fiber; however, extreme pressure, trauma, or ischemia can produce global myofiber necrosis. Necrosis is commonly triggered by increased intracellular calcium concentration, often released from high levels stored in the sarcoplasmic reticulum. Initially, segmental changes are represented by myofiber hyper-contraction, and cross sections appear large and dark with hyalinization and loss of cross striations. Further insult results in sarcoplasmic fragmentation that can lead to dystrophic myofiber mineralization, often seen in chronic myopathies.3,11
Skeletal myofibers are classified as permanent cells and are not capable of cell division. As a result, skeletal muscle regeneration depends on the activation of satellite cells, normally resting between the sarcolemma and the basement membrane. These cells are highly resistant to injury and are activated by necrosis of adjacent myofibers.3,11 Satellite cells begin proliferation and differentiation into myoblasts in the early stages of skeletal muscle regeneration. Concurrently, macro-phages migrate from the peripheral blood and phagocytose necrotic debris leaving a potential space within the damaged muscle. The initial infiltrating macrophages are of the M1 inflammatory phenotype but later switch to the M2 anti-inflammatory phenotype.11 If the basement membrane is intact, the potential space is filled by a scaffold, called the sarcolemmal tube, which prevents the local migration of fibroblasts and instead acts as a guide for proliferating myoblasts. Within the sarcolemmal tubes, satellite cells, known as activated myoblasts at this stage, can be observed undergoing mitoses. Within hours, sarcolemmal tubes fuse end-to-end and form myotubes that eventually mature into skeletal myofibers over the course of a few days. Initial infiltrating M1 macrophages are tough to stimulate proliferation of the myoblasts, while M2 macrophages promote the formation of the myotubes.3,11
In contrast, if large enough numbers of satellite cells are killed and if the basement membrane is destroyed, the sarcolemmal tube is not formed, and there is no proliferation of myoblasts. This allows the influx of fibroblasts into the areas of necrosis resulting in healing by fibrosis rather than regeneration. Additionally, in cases where there is disruption of the basement membrane but satellite cells are still viable, regeneration is disorganized and ineffective due to disorganization of proliferating myotubes. This is typified by the presence of muscle giant cells (large, pleomorphic multinucleated giant myoblastic cells) and fibrous connective tissue.3,11
References:
1. Anderson TD, Van Alstine WG, Ficken MD, Miskimins DW, Carson TL, Osweiler GD. Acute monensin toxocosis in sheep: Light and electronmicroscopic changes. Am J Vet Res. 1984; 45:1142-1147.
2. Bourque JG, Smart M, Wobeser G. Monensin toxicity in lambs. Can Vet J. 1986; 397-399.
3. Cooper BJ, Valentine BA. Muscle and tendon. In: Maxie MG ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier; 2016:180-185.
5. Gregory DG, Vanhooser SL, Stair EL. Light and electron microscopic lesions of broiler cickens due to roxarsone and lasalocid toxicoses. Avian Dis. 1995; 39:408-416.
7. Jones A. Monensin toxicosis in 2 sheep flocks. Can Vet J. 2001; 42:135-136.
8. Mendes O, Mohamed F, Gull T, de la Concha Bermejello. Monensin poisoning in a sheep flock. Sheep and Goat Res J. 2003; 18: 109-113.
9. Novilla MN. The veterinary importance of the toxic syndrome induced by ionophores. Vet and Hum Toxicol. 1992; 34: 66-70.
10. Nation PN, Crowe SP, Harries WN. Clinical signs and pathology of accidental monensin poisoning in sheep. Can Vet J. 1982; 23: 323-326.
11. Valentine BA. Skeletal muscle. In: McGavin MD,ed. Pathologic basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier Mosby; 2017:922-926.