Signalment:
Gross Description:
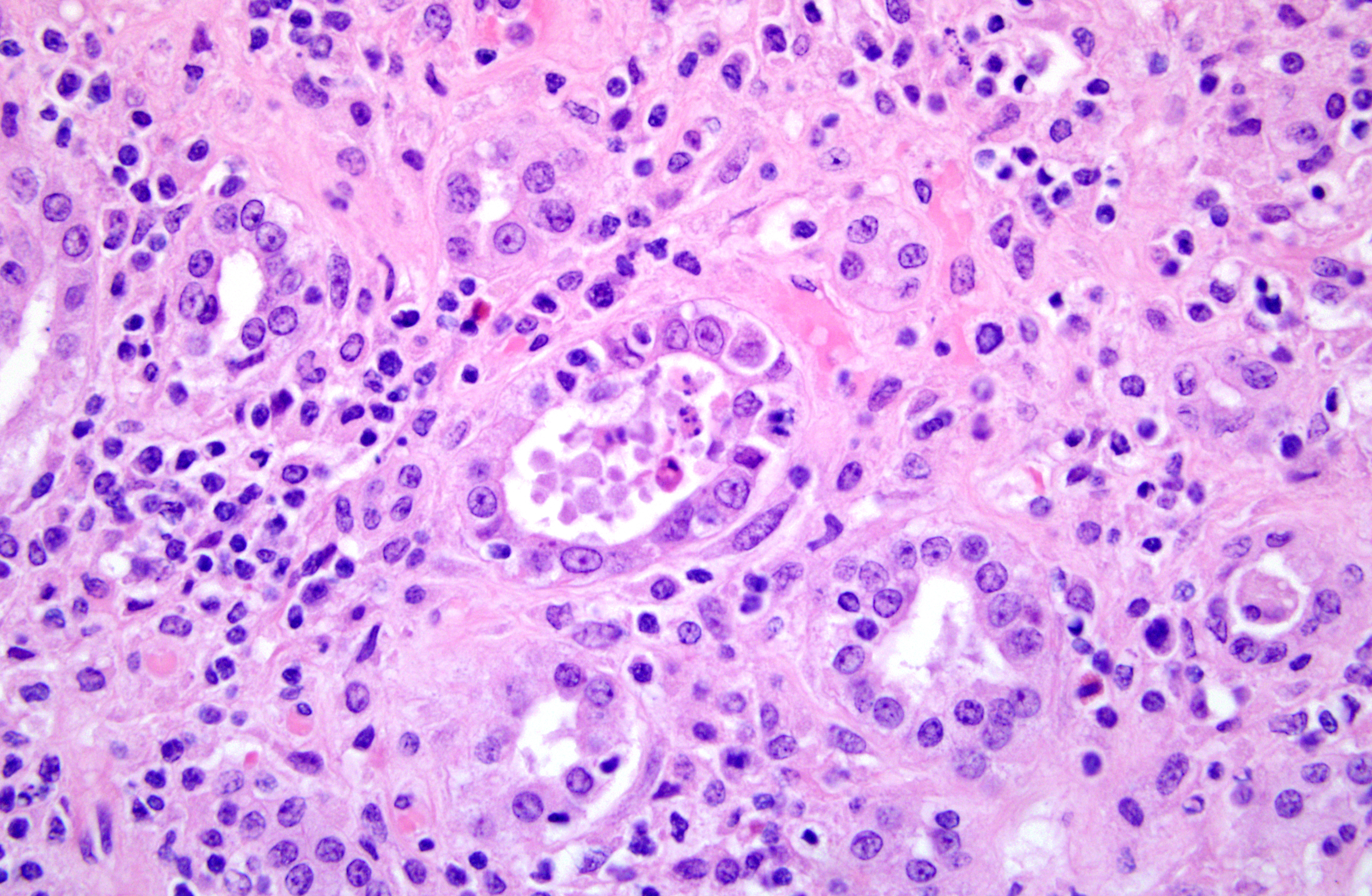
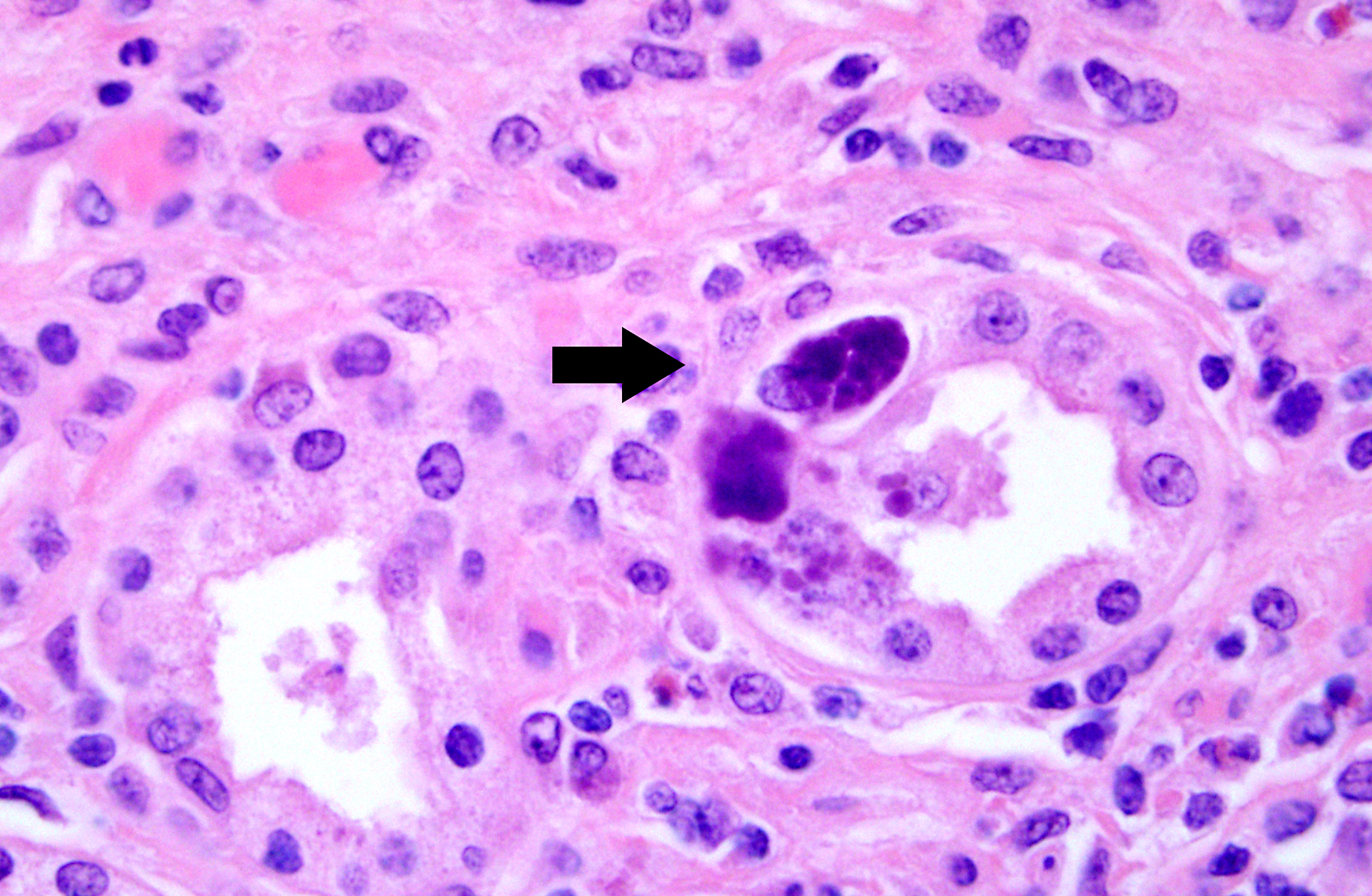
Histopathologic Description:
Morphologic Diagnosis:
1. Kidney. Tubulointerstitial nephritis, lymphoplasmacytic and macrophagic, multifocal, severe, chronic with fibrosis and tubular loss, degeneration, necrosis, and regeneration, and botryoid intraepithelial cytoplasmic inclusions (etiology consistent with porcine circovirus type II infection)
2. Kidney. Arteritis, proliferative and perivascular, lymphocytic, multifocal, severe, chronic with intimal and tunic medial proliferation
3. Kidney. Intravascular coccoid bacteria, multifocal, acute
Lab Results:
Bacteriology: Streptococcus sues and Arcanobacterium pyogenes were isolated from lung. Kidney was not cultured (only fixed renal tissue was received)
Mycoplasma hyopneumoniae PCR was negative (lung)
Virology:
PRRSV PCR was negative (lung)
SIV PCR was negative (lung)
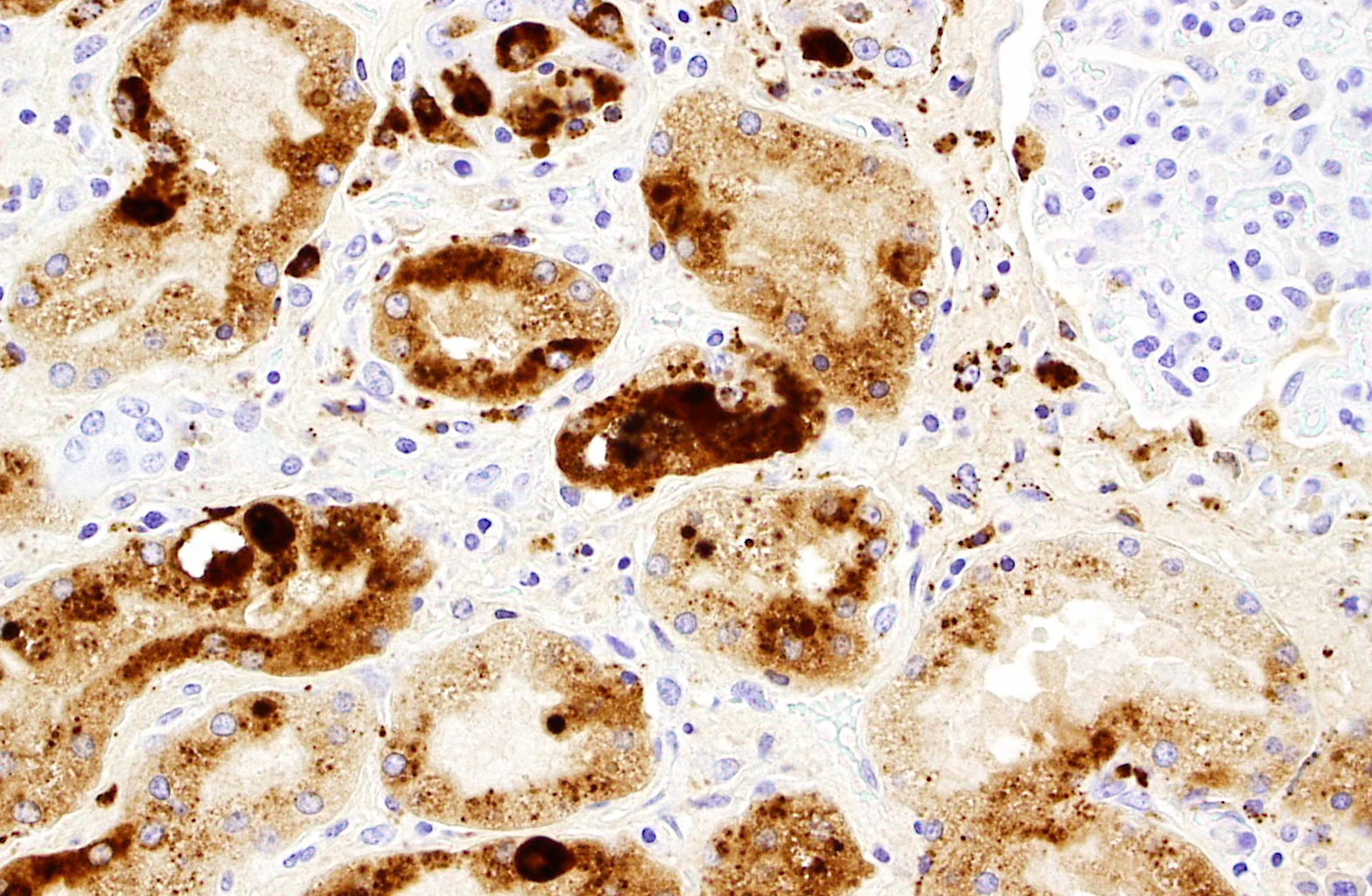
PCV2 IHC was positive (abundant staining, in kidney, spleen, and lymph node)
Condition:
Contributor Comment:
Subclinical disease: PCV2 can be detected in normal, healthy pigs without signs of disease. Subclinical PCV2 infection can reduce growth performance, can cause increased susceptibility to other swine-associated pathogens, or result in decreased efficacy of commonly administered swine vaccines(7). Quantification of PCV2 DNA in serum and tissue has been proposed as a means of differentiating subclinical from clinically affected animals. Subclinically infected PCV2 pigs typically have lower amounts of DNA in serum and tissue (â¥107 genomic copies per ml/serum or tissue).
Post-weaning multisystemic wasting syndrome (PMWS): PMWS is a multifactorial systemic disease and clinically manifests at 25-150 days of age with most cases occurring between 7 and 15 weeks. Six fundamental clinical signs are emphasized and include wasting, dyspnea, lymphadenopathy, diarrhea, pallor and jaundice. Coughing, pyrexia, gastric ulceration, and meningitis have also been reported, but are sporadic. Not all clinical signs have to be present in an individual pig, but all six fundamental signs can generally be observed among the population of pigs in affected herds over time.(4) Herd morbidity in PMWS herds is variable with 4-30% of the pigs affected. Mortality rates are often high (20%) with occasional reports of greater than 50% death loss within a group. The most consistent necropsy finding in PMWS pigs is generalized lymph node enlargement. Other macroscopic lesions can include mottled tan non-collapsing lungs and thymic atrophy. PMWS pigs are frequently coinfected with other common bacterial and viral pathogens, and coinfection often complicates gross findings.
Microscopic lesions in PMWS-affected pigs are consistently found in lymphoid tissues and characterized by loss (depletion) of lymphocytes and replacement by histiocytic/granulomatous inflammation. Lymphoid depletion can be associated with paracortical or follicular regions with the latter being more prevalent. Multinucleated giant cells (Langhans-type), epithelioid macrophages and macrophage-associated intracytoplasmic, botryoid-like, basophilic inclusions are also commonly seen in lymphoid tissues (lymph node, tonsil, and spleen). Focal parenchymal coagulative and apoptotic necrosis in lymphoid tissues has also been described. Microscopic lesions in nonlymphoid tissues are composed of lymphomacrophagic inflammation and include interstitial pneumonia, hepatitis, interstitial nephritis and enteritis/colitis.
Clinical signs along with macroscopic and microscopic lesions associated with PCV2 infection are suggestive, but not definitive for a PMWS diagnosis in an individual or group of pigs. A definition diagnosis of PMWS is based on the following definition: (1) clinical signs of wasting, weight loss or failure to thrive that may or may not include respiratory distress and icterus, (2) microscopic lesions of lymphoid depletion and granulomatous inflammation, and (3) the presence of PCV2 antigen or nucleic acid associated with microscopic lesions.(8) Not all PMWS-affected pigs will have equal distribution of microscopic lymphoid lesions.
PCV2-associated respiratory disease: PCV2-associated respiratory disease can often overlap with PMWS or be a contributing factor in porcine respiratory disease complex (PRDC). In the first cases of PMWS, lymphomacrophagic interstitial pneumonia was a common microscopic lesion. Other hallmark microscopic lung lesions of PCV2 include type II pneumocyte hypertrophy and hyperplasia, peribronchiolar fibrosis, and associated lymphoid hyperplasia.(2)
PCV2-associated enteritis: The suspected major route of PCV2 transmission is fecal-oral, suggesting that the alimentary mucosa, intestinal M-cells, and gut-associated lymphoid tissue (GALT; Peyers patches) are exposed to varying amounts of infectious virus. Peyers patches can exhibit hallmark microscopic lesions of lymphoid depletion and granulomatous inflammation in both PCV2-associated enteritis and PMWS-affected pigs. The diagnosis of
PCV2-associated enteritis is appropriate when (1) there is clinical diarrhea, (2) the hallmark microscopic lesions are present in Peyers patches but not in other lymphoid tissues, and (3) PCV2 DNA or antigen can be demonstrated within the lesions.(2)
PCV2-associated enteritis may occur anytime in the grow-finish phase, and can be associated with the small or large intestine. Affected intestinal segments are sometimes thickened and necrotic resembling gross lesions of Lawsonia intracellular is infection and can be misdiagnosed as such. Microscopically, PCV2-associated enteritis has been described as having variable amounts of macrophages infiltrating the mucosa with PCV2 antigen present in crypt epithelium, the lamina propria, and submucosa. Villous atrophy and fusion along with multinucleated giant cells within the lamina propria have also been described.
Porcine dermatitis and nephropathy syndrome (PDNS): PDNS is a distinctive, acute clinical entity of growing swine that was first recognized in 1993 and is now globally distributed.(2) Affected pigs have circular to coalescing, red to purple macules or raised papules and plaques, occasionally with black centers, that originate on the skin of the hind legs and perineal region. Lesions may become exudative, crust-over, and eventually regress leaving dermal scars. Bilateral swollen kidneys with widely disseminated cortical petechial hemorrhages are common features of the syndrome. Increased mortality is seen in affected pigs older than three months of age, the syndrome is sporadic, and herd-outbreak mortality can range from 0.25 to > 20%.
The hallmark microscopic lesion in PDNS-affected pigs is necrotizing vasculitis and glomerulonephritis. Small to medium sized dermal and subcutaneous arterioles are cuffed by neutrophils, macrophages, lymphocytes, and plasma cells that are sometimes present within vascular walls. Arterioles are lined by plump endothelial cells, occasionally occluded by fibrin thrombi and walls can display multifocal hyalinization. The corresponding dermis is necrotic and hemorrhagic. Kidney sections are characterized by distension of urinary spaces by fibrin intermixed with necrotic cellular debris and hemorrhage, periglomerular and interstitial mononuclear cell infiltration, and distension of renal tubules that contain cellular and proteinaceous casts. Perirenal lymph nodes are sometimes depleted and hemorrhagic.
Skin and glomerular lesions are characteristic of a type III hypersensitivity reaction with deposition of antigenantibody complexes (immune complexes). Immune complexes have been demonstrated in glomerular tufts. Multiple viral and bacterial pathogens have been implicated in PDNS, but PCV2 is considered a contributing. To date, PDNS has not been reproduced with PCV2 infection.
JPC Diagnosis:
1. Kidney: Nephritis, interstitial, lymphohistiocytic, diffuse, moderate, with tubular degeneration, necrosis, and regeneration, granular and cellular casts, intraepithelial intracytoplasmic botryoid inclusions, and proliferative, eosinophilic and histiocytic arteritis.
2. Kidney, glomeruli and vasa recta: Intravascular bacterial emboli.
Conference Comment:
A central nervous system manifestation of PCV2 infection is cerebellar hemorrhage and edema from necrotizing and lymphohistiocytic vasculitis and thrombosis. Gross findings include multiple cerebellar petechiae and fibrinopurulent meningitis. PCV2 antigen has been demonstrated in macrophages and cerebellar endothelial cells in affected areas(3).
A differential diagnosis discussed by conference participants is leptospirosis, which results in clinical illness in only a small portion of infected swine. The primary effect on swine is abortion and the birth of weak piglets, but leptospirosis may also result in interstitial nephritis and renal papillitis with infiltration by mononuclear cells and numerous bacteria in the medulla(6).
References:
2 Chae C: A review of porcine circovirus 2-associated syndromes and diseases. Vet J 169:326-336, 2005
3 Correa AM, et al. Brain lesions pigs affected with postweaing multisystemic wasting syndrome. J Vet Diagn Invest. 2007; 19:109-12.
4 Harding JC: The clinical expression and emergence of porcine circovirus 2. Vet Microbiol 98:131-135, 2- 4-2004
5 Huang YY, Walther I, Martinson SA, Lopez A, Yason C, Godson DL, Clark EG, Simko E: Porcine circovirus 2 inclusion bodies in pulmonary and renal epithelial cells. Vet Pathol 45:640-644, 2008
6 Maxie MG, Newman SJ. Urinary System. In: Maxie MG, ed. Jubb, Kenedy, and Palmers Pathology of Domestic Animals. 5th ed. Philadelphia, PA:Saunders Elsevier; 2007:487-9.
7 Opriessnig T, Meng XJ, Halbur PG: Porcine Circovirus Type 2 associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:591- 615, 2007
8 Sorden SD: Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Hlth Prod 8:133-136, 2000