Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 10
28 November 2018
CASE IV: 15-H700012 (JPC 4084546).
Signalment: 21 week old, intact male, domestic rabbit (Oryctolagus cuniculus)
History: 1 day history of sneezing with acute onset on respiratory distress.
Gross Pathology: The animal was in poor nutritional condition characterized by a near-complete lack of subcutaneous, perirenal and mesenteric adipose tissue. The right nasal cavity was nearly completely filled with a tan, viscous material.
Laboratory results: None given
Microscopic Description:
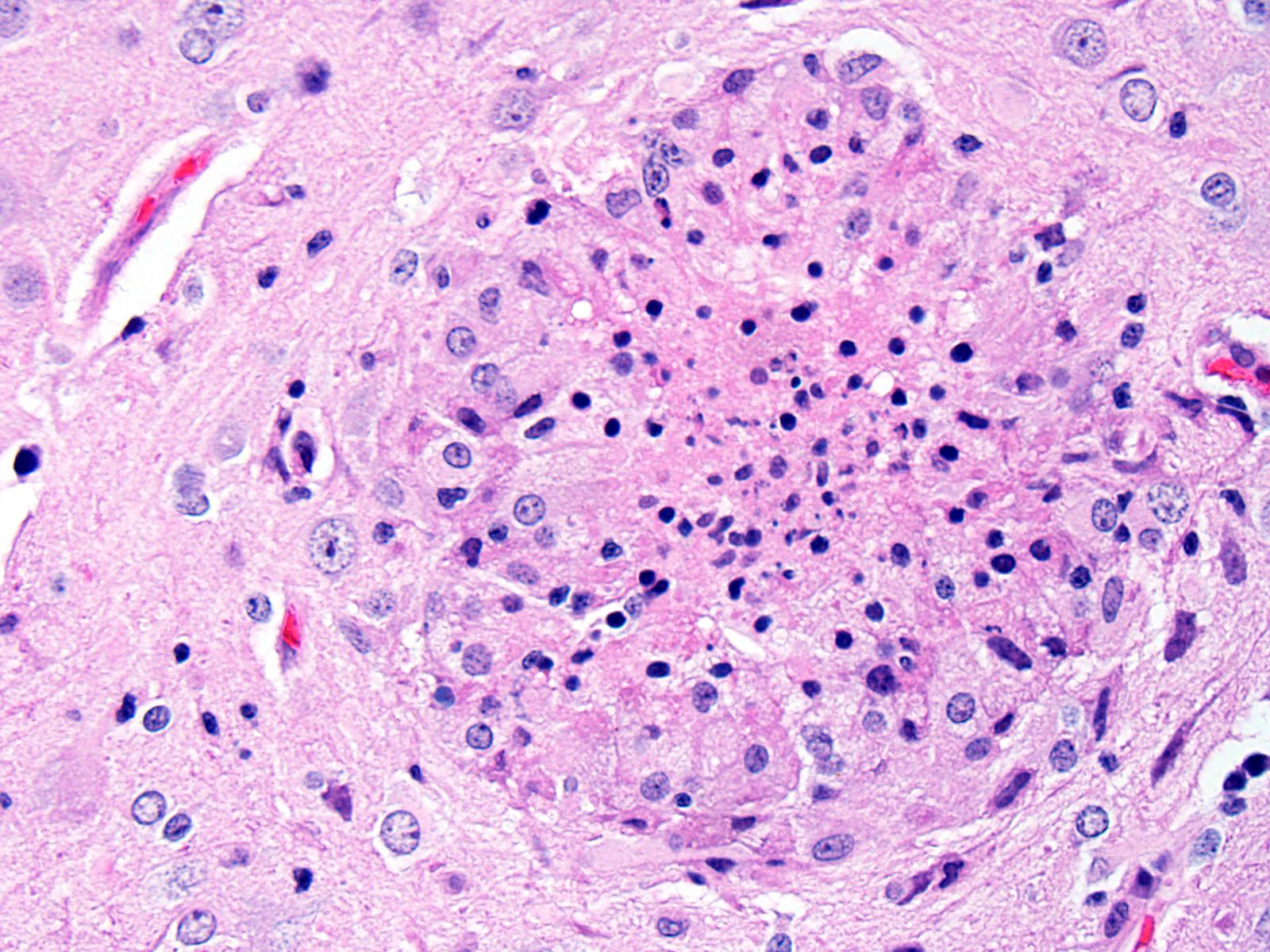
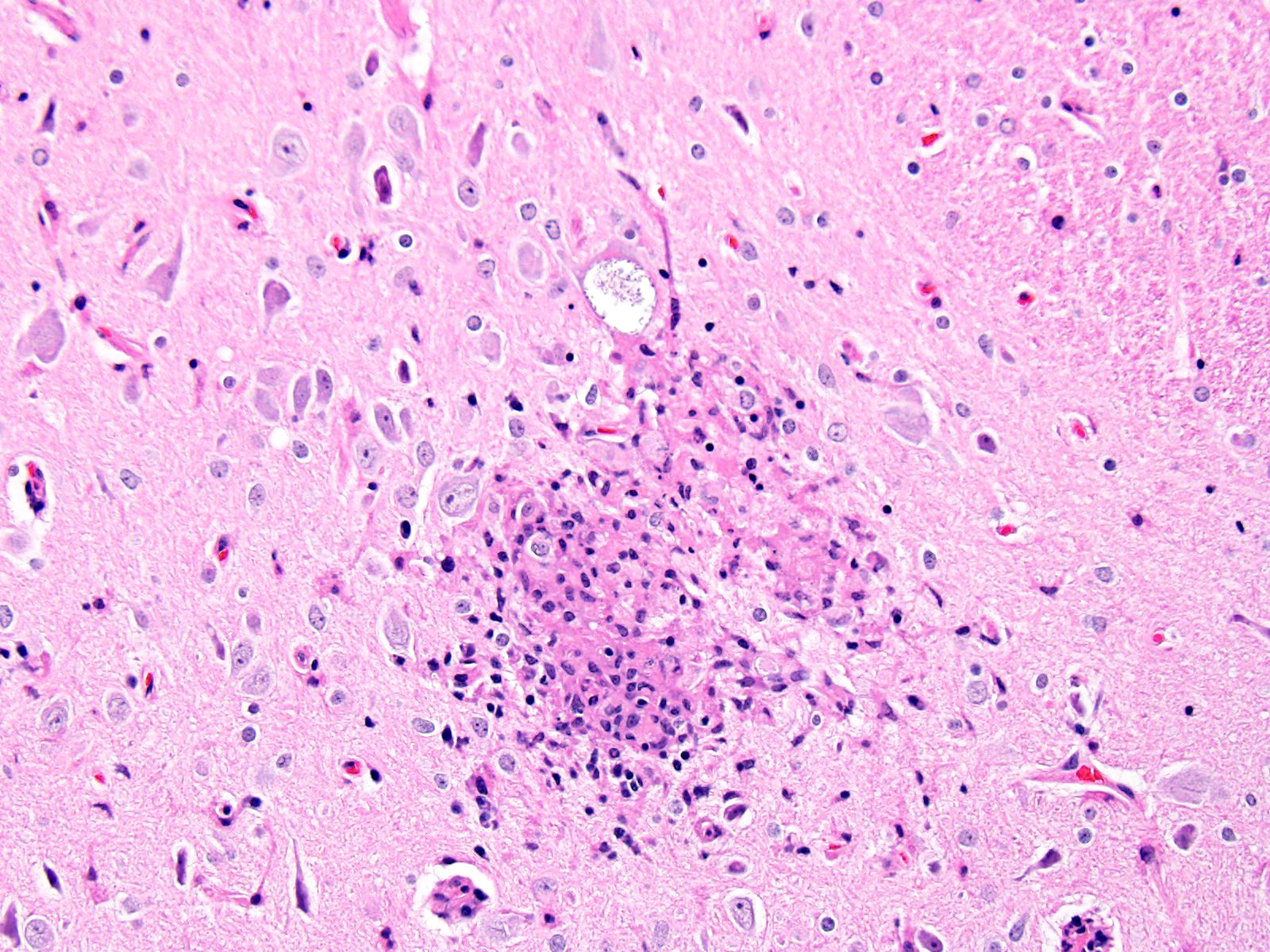
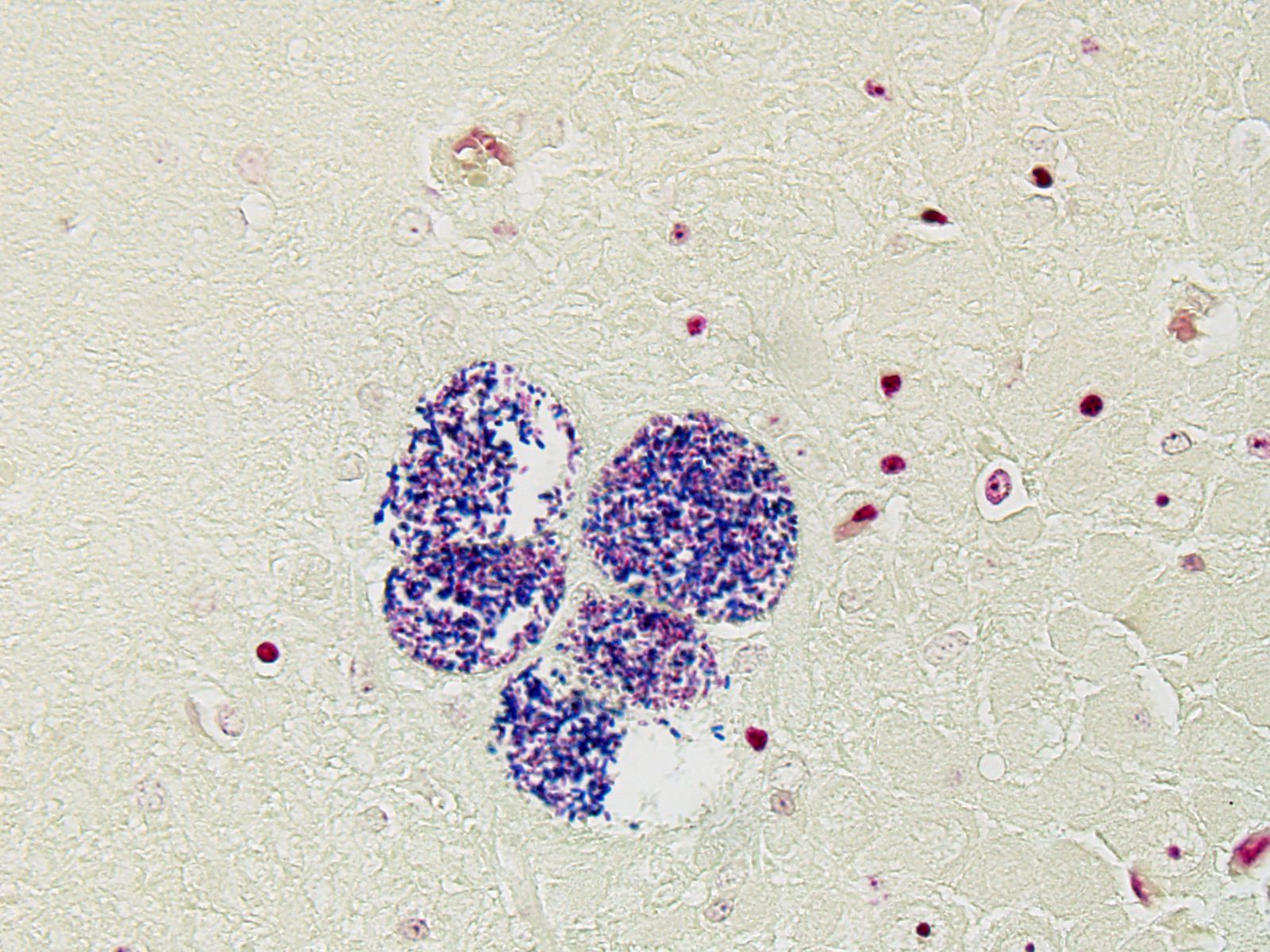
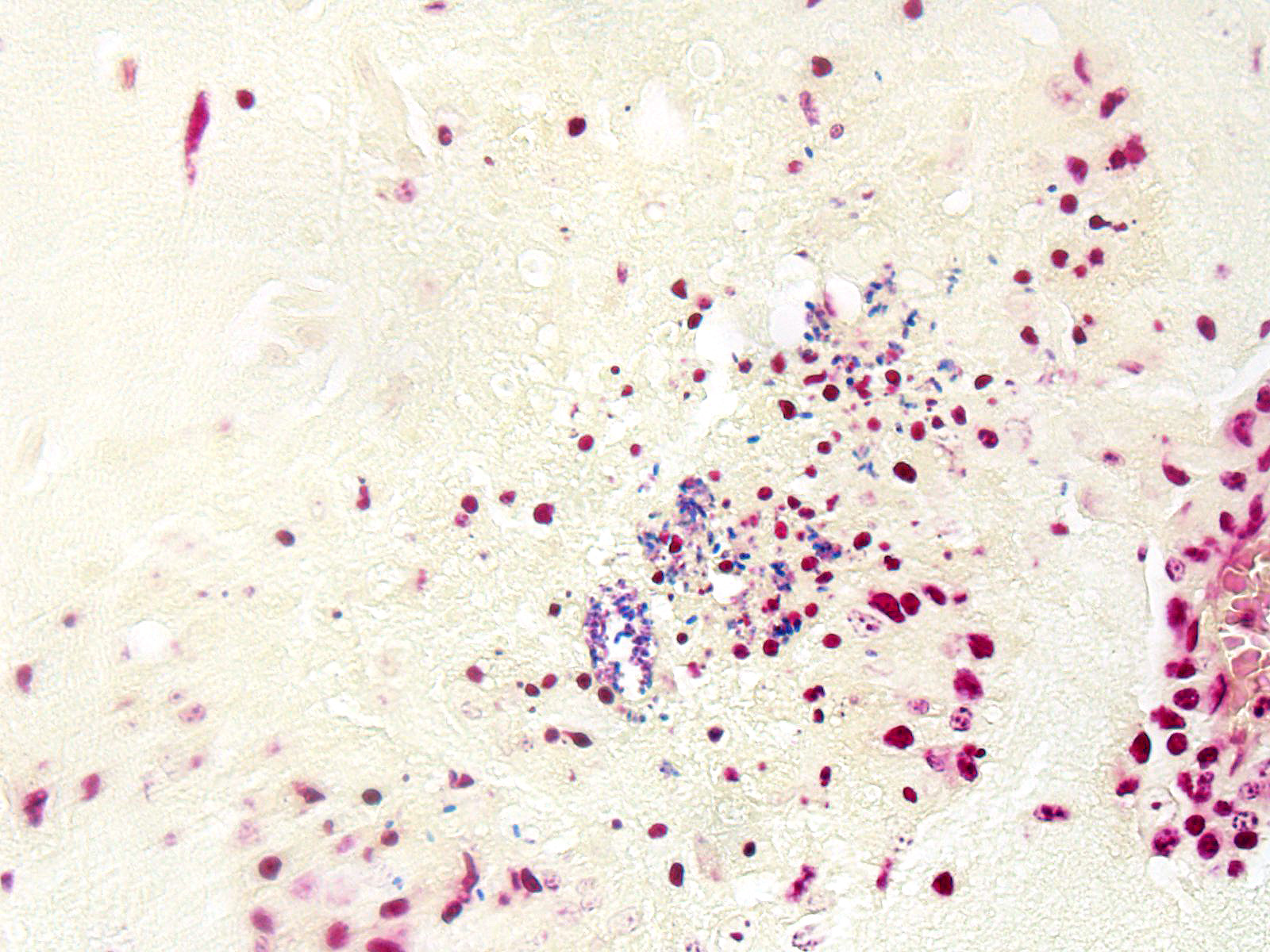
BRAIN (Some slides contain cerebrum while others contain hippocampus and cerebellum, but the lesions are identical): Infiltrating both grey and white matter, there are multiple aggregates of epithelioid macrophages with lesser numbers of lymphocytes and plasma cells and rare heterophils. The inflammatory infiltrates frequently surround accumulations of eosinophilic cellular and karyorrhectic nuclear debris (necrosis). Occasional necrotic foci contain irregular, fragmented, basophilic material (mineral). Within inflammatory foci, there are rare, 20-30 µm pseudocysts that contain numerous 1x3 µm refractile spores with a small, basophilic nucleus. The neuropil surrounding the necrotic and inflammatory foci contains moderate numbers of reactive glial cells (gliosis). Vascular endothelial cells are frequently plump (reactive), and Virchow-Robin spaces often are expanded with macrophages, lymphocytes and plasma cells. The leptomeninges are expanded with moderate numbers of lymphocytes and plasma cells.
Contributor’s Morphologic Diagnoses:
Brain (cerebrum, hippocampus, and cerebellum): Meningoencephalitis, granulomatous and necrotizing, multifocal with microsporidial spores (Encephalitozoon cuniculi).
Contributor’s Comment: Wright and Craighead initially identified Encephalitozoon cuniculi in 1922 as the agent responsible for “infectious motor paralysis” in rabbits exhibiting lethargy, tremors and paresis.14 E. cuniculi are members of the phylum Microspora, which contain a diverse collection of obligate intracellular organisms which can exist as environmentally resistant spores outside of the host.12 There has been considerable debate and reclassification with regards to the evolutionary origin of Microspora. These intracellular eukaryotes lack mitochondria and peroxisomes, and were originally thought to belong to a deeply branching protist lineage diverging prior to the emergence of the mitochondria as an endosymbiont (11). Modern phylogenetic analysis reveals that Microsporidia are actually more closely related to the fungal domain.5 Phenotypically, the defining criterion for inclusion is the phylum is an organelle found coiled within the spore termed a “polar filament” or a “polar tube”.1
Besides E. cuniculi, two other species with the genus Encephalitozoon are also known to infect mammals: Encephalitozoon hellum and Encephalitozoon intestinalis.8 E. cuniculi is capable of infecting a wide range of mammalian hosts, including rabbits, rodents, horses, carnivores and humans.7 In humans, E. cuniculi and other microsporidia have been identified as opportunistic pathogens in immunocompromised patients, though not causing the same epidemiological human morbidity and mortality as other parasites such as Plasmodia, Trypanosoma, Leishmania and Toxoplasma gondii. At one time, E. cuniculi was thought to be the agent responsible for rabies and polio.12
The main host for E. cuniculi is the rabbit, and infections are mostly subclinical, and the course is typically chronic, taking weeks to months to develop a parasite burden sufficient to cause clinical signs.7 The seroprevalence in pet populations is high (between 37% and 68%) due to proximity.4 Historically, in laboratory rabbit colonies, E. cuniculi was a significant problem that resulted in interference with experiments and affected the overall health of the colonies.10 Generally, infected rabbits will exhibit the non-specific signs of weight loss and failure to thrive, but can also manifest neurological signs such as ataxia, opisthosomas, torticollis, hyperesthesia, or paralysis.6 Phacoclastic uveitis is also possible, and is characterized by a mixed inflammatory response (granulocytes, macrophages and multinucleate giant cells) causing lens capsule rupture, with organisms found only within the lens.3
In naturally infected rabbits, transmission occurs through organisms shed in the urine that are orally consumed, although transplacental infections are documented.9,12 In experimental conditions, rabbits are also capable of being infected via a respiratory route in addition to oral. Following exposure, spores infect mononuclear cells and thereby enter they systemic circulation via leukocyte trafficking.9 The first organs affected are the lung, liver and kidney, but later (approximately 3 months post-exposure), the lesions in the lung and liver subside and there are significant changes to the brain as well as the kidneys.7 Gross lesions are not typically seen, although rarely there can be focal, irregular and depressed regions in the renal cortex.2
Reliable histological lesions in the brain consist of focal to multifocal, non-suppurative meningoencephalitis with gliosis and perivascular accumulations of lymphocytes and plasma cells. In some instances, there can be foci of necrosis surrounded by epithelioid macrophages with lesser numbers of lymphocytes and plasma cells.13 Lesions in the kidneys include focal to segmental interstitial nephritis with tubular epithelial degeneration, necrosis and sloughing. There is little to no glomerular involvement.9,13 Spores can be identified using Gram stain an ovoid, 1.5 x 2.5-5 um diameter structures, which also stain purple with carbol fuchsin.9
Contributing Institution:
University of Illinois College of Veterinary Medicine, Department of Pathobiology, 2522 Veterinary Medicine Basic Science Building, 2001 S. Lincoln Ave., Urbana, IL
61802 http://vetmed.illinois.edu/research/departments/pathobiology/
JPC Diagnosis: Cerebrum: Encephalitis, granulomatous and necrotizing, multifocal, moderate with lymphoplasmacytic meningitis and numerous microsporidian spores.
JPC Comment: Microsporidia are unique organisms which apparently have evolved from fungi, being most closely related to the zygomycetes.7 Other similarities between microsporidia and fungi include the presence of chitin and trehalose, similarities between the cell cycles, and the organization of certain genes. Other unique factors include their lack of mitochondria (although enzymes with mitochondrial functions have been conserved), and ribosomal RNA which more closely resembles that of prokaryotes than eukaryotes. Particularly impressive is their extremely small genome, comprising 2.9 Mbp, which has been accomplished due to the compression of genetic information within chromosomes, resulting from a virtual absence of introns, a low number of repetitive sequences and many single-copy genes, an overall shortening of genes as well as of non-coding sequences, as well as the absence of genetic information regarding certain metabolic pathways that are unnecessary for the organism’s parasitic lifestyle.7,8
The unicellular spores of these parasites are unique within the animal kingdom with incorporation of the nucleus, the posterior vacuole, and ribosomes into “sporoplasm”, which is injected into new host cells by virtue of a polar tube, a specialized invasion apparatus which works much like a hypodermic needle. In addition, the polar tube may stain positively with a number of histochemical stains, including modified trichrome, Luna, PAS, and Giemsa facilitating identification of microsporidia in tissue section.
- cuniculi may parasitize a wide range of mammals, but is most commonly associated with infections in rabbits, rodents, dogs, and primates. Based on the number of short repeats in the ribosomal internal transcribe spacer region, three different strains of E. cuniculi of have been identified: the “rabbit” (type I), the “mouse (type 2), and the “dog” stain (type 3). These strains are useful in identifying the source of human outbreaks (with only infections with dog and rabbit strains being previously identified). Outbreaks in rabbits appear to occur solely from rabbit strains.8
The contributor has an excellent job in detailing infection in the rabbit. While lesions in the rabbit are classically considered to be limited to the CNS and kidney, acute infections may results in cysts and spores within the lung, liver and heart, sites which are clear of organisms when the clinical signs of neurologic disease or renal failure become evident. Lenticular invasion is a particularly interesting phenomenon which appears to be restricted to dwarf rabbits; unlike normal horizontal infections, this syndrome results from vertical transmission to the developing lens via a peculiar homing mechanism for E. cuniculi to the developing optic cup where it takes residence within the lens. Delayed replication results in destruction of lens fibers and leaching of lens protein into the globe, ultimately causing severe granulomatous inflammation and phthisis.
Infections with Encephalitozoon sp. and a related microsporidian genus, Enterocytozoon, have been reported in humans. While most cases of infection of E. cuniculi are associated with severe immunodeficiency, a number of cases have occurred in immunocompetent individuals in close contact with rabbits and dogs. As spores of E. cuniculi are resistant within the environment, waterborne infections could not be totally excluded in such cases.8 Other related species of Encephalitozoon, to include E. hellem and E. intestinalis, are also well reported in the literature. E. hellum, is a microsporidian parasite primarily of psittacine birds which has been almost exclusively diagnosed in HIV-infected individuals. E. intestinalis is the second most prevalent microsporidian species infecting humans and is a common cause of diarrhea and other gastrointestinal complaints in HIV-infected individuals.8
A related microsporidia parasite, the most common species known to cause human disease, is Enterocytozoon bienusi. This pathogen was first described in HIV-infected patients in 1985 and may be found in up to 50% of HIV infected patients.8 It has also been identified in immunocompetent patients associated with traveler’s diarrhea in Europe. E. bienusi has also been identified in immunocompetent diarrhea patients in concert with E. hellem and E. intestinalis.8 Over the last decade, evidence has accumulated that this parasite may persist as an asymptomatic infection in immunocompetent humans. Eleven years after its discovery as a human pathogen, Enterocytozoon was detected in animals for the first time and seems to be a common parasite in asymptomatic pigs.8 It has also been reported as a common finding in SIV-infected macaques, but may also be seen in immunocompetent macaques as well. It has not been documented in macaques in the wild.8
The determination of the morphologic diagnosis in this case was met with spirited discussion, and other descriptive features of the morphology of this disease were also greeted with some skepticism (including the oft-used term “pseudocyst”). Granulomatous encephalitis is a classic description of the inflammation associated with cerebral encephalitozoonosis (and is used in some very recent textbooks on laboratory animal disease, yet the true composition of the inflammatory foci surrounding extracellular spores is difficult to elucidate on morphologic grounds alone, and a more in-depth analysis of cell types contained in these foci is not available. The lack of multinucleated cells, lymphocytes, and plasma cells in inflammatory foci do not support granulomatous inflammation, and pathologists who refer to these foci as “glial nodules” may in fact be closer to the truth. Our morphologic diagnosis above is a combination of tradition and morphology – granulomatous on faith to excellent morphologists who preceded us, and necrotizing on evidence.
References:
- CanningEU,Lom J: The Microsporidia of Vertebrates. Harcourt Brace Jovanovich, New York, NY, 1986.
- FlattRE, Jackson SJ: Renal nosematosis in young rabbits. Vet Pathol. 1970;7:492–497.
- Giordano C, Weigt A, Vercelli A, Rondena M, Grilli G, Giudice C. Immunohistochemical identification of Encephalitozoon cuniculiin phacoclastic uveitis in four rabbits. Vet Ophthalmol. 2005;8:271–275.
- Harcourt-Brown FM, Holloway HKR (2003)Encephalitozoon cuniculiin pet rabbits. Vet Rec. 2003;152:427–431.
- Katinka, MD, et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature, 2001;414, 450-453.
- Kunstyr I, Naumann S: Head tilt in rabbits caused by pasteurellosis and encephalitozoonosis. Lab Anim. 1985;19: 208–213.
- Kunzel F, Joachin A: Encephalitozoonosis in Rabbits. Parasitol Res. 2010;106:299-309.
- Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin Microbiol Rev. 2005;18:423–445.
- Percy DH, Barthold SW, Griffey SM. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Blackwell Publishing; 2016: 293-295.
10.Shadduck JA. Effect of fumagillin on in vitro multiplication of Encephalitozoon cuniculi. J Protozool. 1980;27(2):202–208.
- Vossbrinck, C. R., Maddox, J. V., Friedman, S., Debrunner-Vossbrinck, B. A. & Woese, C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes.Nature, 1987;326, 411-414.
- Wasson K, Peper RL. Mammalian Microsporidosis. Vet Pathol. 2000; 37(2): 113-128.
- Wicher V, Baughn RE, Fuentealba C, Shadduck JA, Abbruscato F, Wicher K (1991) Enteric infection with an obligate intracellular parasite,Encephalitozoon cuniculi, in an experimental model. Infect Immun. 1991; 59:2225–2231.
- Wright JH, Craighead EM. Infectious motor paralysis in young rabbits. J Exp Med. 1922;36:135–140.