CONFERENCE 25, CASE 2
Signalment:
1 year, 2 months old male sitatunga
History:
This sitatunga (Tragelaphus spekii) was born at a zoological institution in the mid-Atlantic region. He was apparently healthy at birth, nursed well, and had normal baseline bloodwork. He received routine vaccinations for tetanus at 8 and 12 weeks of age and rabies at 16 weeks. At 3 months of age, he was first reported to have mild, intermittent ataxia in the rear limbs, which continued over the next several months, along with occasional rear limb lameness. There were periods when the ataxia became more severe and the animal became lethargic; at times signs progressed to leg crossing, circling and falling. He was somewhat responsive to treatment with NSAIDs and antibiotics, but intermittent ataxia persisted. Bloodwork was always within normal limits and spinal radiographs were unremarkable. Vitamin E serum levels were slightly low but similar to other animals in the collection that did not have clinical signs. He was treated with injectable vitamin E/Selenium weekly with some improvement in ataxia. At 11 months of age the animal presented with acute worsening of ataxia, with a right head tilt, right circling and collapsing, horizontal nystagmus, and crossing of legs in front and back. Over the next two months he was treated with NSAIDs, vitamin E/selenium and showed mild improvement. On the day prior to necropsy, he acutely became severely ataxic, with crossing of front and rear legs, right head tilt, severe right circling leading to falling, and decreased awareness of surroundings. Euthanasia was elected.
Gross Pathology:
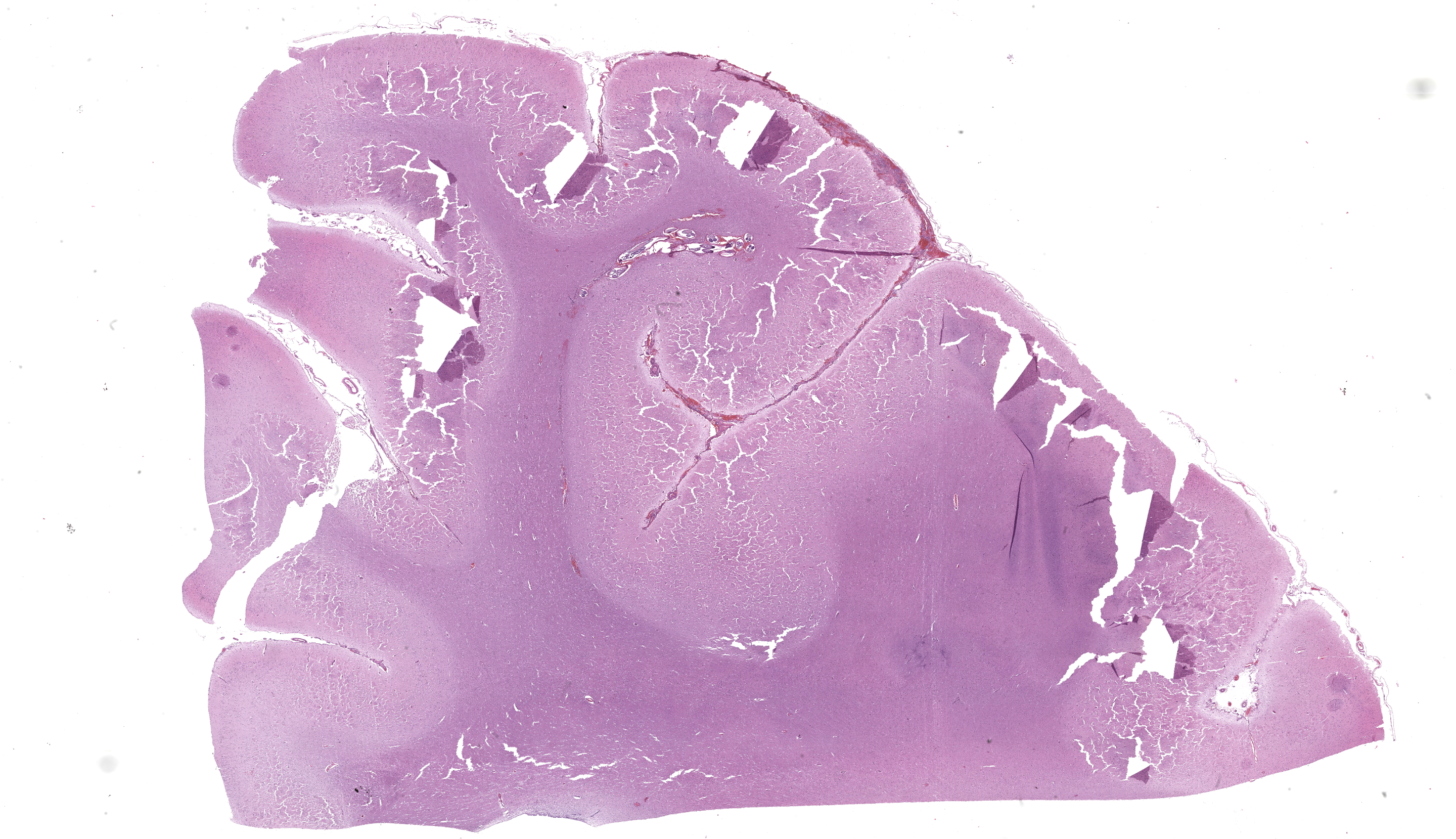
Post-fixation, on cut section there was an area of hemorrhage within the parenchyma at the level of the right basal ganglia, and multiple dark brown areas within the meninges and extending into the superficial cortex
Laboratory Results:
PCR targeting a portion of the rRNA ITS-2 region of Parelaphostrongylus spp was performed on DNA isolated from formalin-fixed brain tissue from this case. The resulting PCR product showed 100% sequence identity to P. tenuis
Microscopic Description:
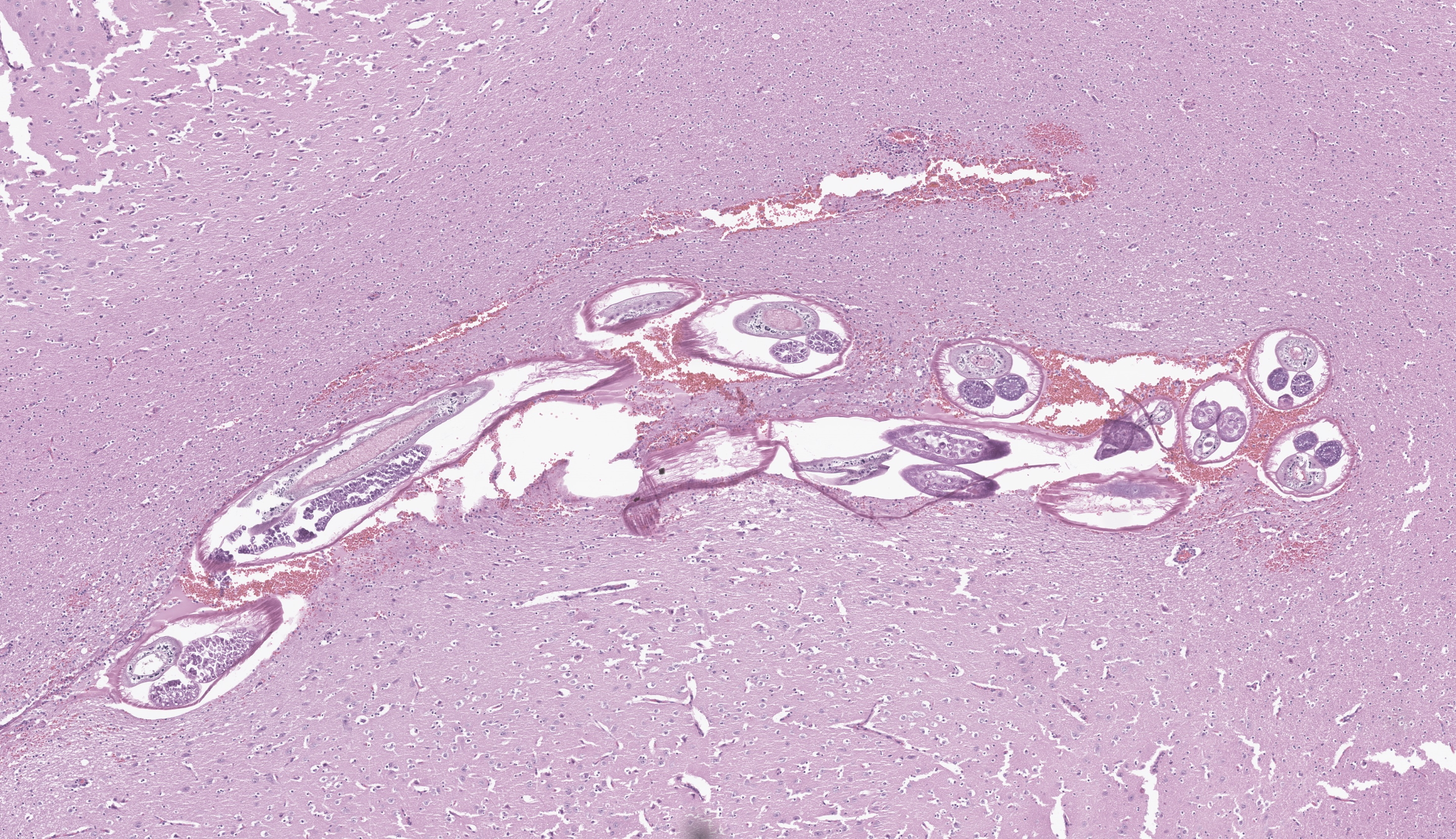
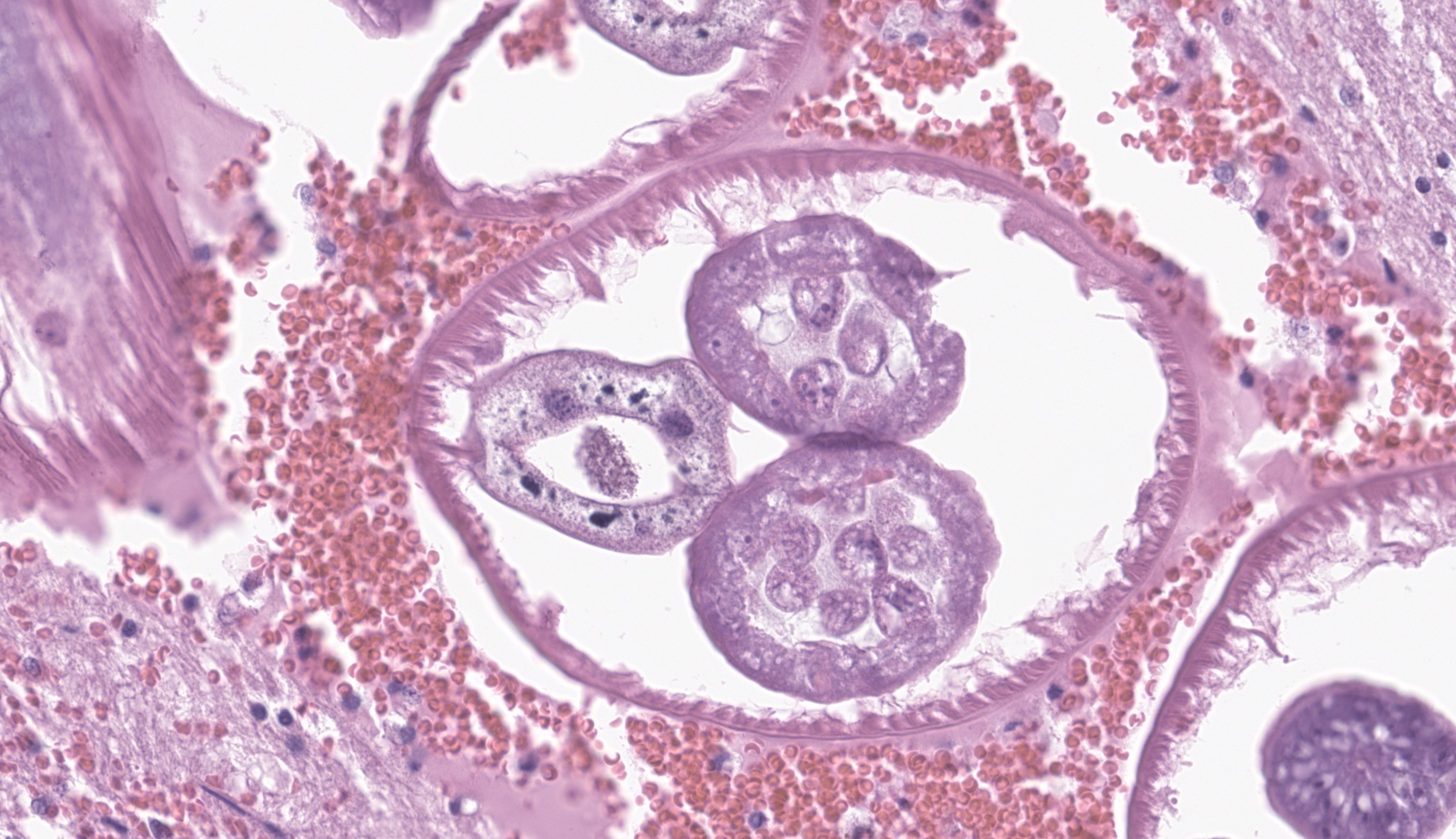
Cerebrum at the level of the basal ganglia: Beneath the meninges and extending into the outer layers of the cerebral cortex is a focally extensive area of hemorrhage, with rarefaction of the neuropil and abundant eosinophils, lymphocytes, histiocytes, and plasma cells. Throughout the parenchyma, there are multiple smaller tracts of hemorrhage, necrosis and loss of neuropil. Within some of these lesions are several, 100-250 um in diameter cross sections of adult nematodes characterized by a thin smooth cuticle, coelomyarian musculature, accessory hypodermal chords, a large intestinal tract with few multinucleate cells, and a reproductive tract. There is moderate to severe periventricular edema and gliosis, and multifocal lymphoplasmacytic perivascular cuffing.
Contributor’s Morphologic Diagnosis:
Brain: meningoencephalitis, lymphohistiocytic and eosinophilic, multifocal, chronic active, severe, with reactive gliosis, hemorrhage, necrosis, and intralesional nematodes
Contributor’s Comment: The necrotizing tracts in the brain caused by migrating nematodes are consistent with the clinical signs of progressive ataxia, circling, head tilt, and loss of awareness of surroundings. Histological characteristics of the organism including a thin smooth cuticle, coelomyarian musculature, accessory hypodermal chords, and a large intestinal tract with few multinucleate cells, are consistent with a metastrongyle nematode.4 PCR and DNA sequencing confirmed identification of Parelaphostrongylus tenuis.
P. tenuis, also known as the meningeal worm, is a nematode most commonly found in white-tailed deer which is the definitive host. In this species, adult worms are found within the cranial venous sinuses and the subdural space. Here, adults lay eggs that travel through the venous blood and into the lungs. In the lungs, eggs embryonate into larvae which move into the respiratory tract, then are swallowed and eliminated into the feces. Larvae then penetrate into the foot of terrestrial molluscs. Deer or aberrant hosts become infected by ingesting gastropods which contain infective larvae. After ingestion, larvae migrate to the spinal cord and develop into adults in the dorsal horn of the gray matter. They migrate into the spinal subdural space and into the cranium, through the dura mater and into the venous sinuses.1
White-tailed deer commonly harbor meningeal worms, particularly in areas of deciduous forest regions where the habitat is suitable for gastropods. Prevalence in white-tailed deer populations is highly variable and has been reported to be up to 94% in certain areas.6
P. tenuis causes little damage to the definitive host, but can cause severe neurologic disease in other animals such as moose, caribou, wapiti, mule deer, fallow deer, elk, llamas, alpacas, wolves, horses, antelope, sika deer, sheep, calves, goat, and guinea pigs.1-3,5-10 Clinical signs can include ataxia, hypermetria, paresis, paralysis, head tilt, circling, blindness, weight loss, depression, seizures, and death. Treatment of mildly affected animals has been successful with anthelminthics such as ivermectin, steroids, and supportive care. However, prognosis is guarded in animals with severe neurological signs.6 To our knowledge, this is the first report of P. tenuis infection in a sitatunga.
Contributing Institution:
Johns Hopkins University, School of Medicine
Department of Molecular and Comparative Pathobiology
Broadway Research Building, #811
733 N. Broadway
Baltimore, MD 21205
Phone: 443-287-2953
Fax: 443-287-5628
http://mcp.bs.jhmi.edu/
JPC Morphologic Diagnosis: Cerebrum: Meningoencephalitis, necrohemorrhagic, focally extensive, subacute, moderate, with adult metastrongyle nematode.
JPC Comment: The contributor provides a lovely section of P. tenuis that illustrates the characteristic features of this nematode in histologic section. While PCR is an important tool in diagnostic and research settings, it is important to recognize these features and generate a putative ‘culprit’ as recovery of nucleic acids, particularly from formalin-fixed tissue can present issues with organism identification. In contrast to the first case, the thickening of the meninges is more pronounced here and is accompanied by both rarefaction (small/fine-sized vacuolar change of the parenchyma) and spongiosis (appreciably larger vacuoles) in the immediate vicinity. The linear tract of necrosis and hemorrhage functions almost as a signpost towards the profiles of these nematodes, though absent these arriving at a specific diagnosis would be difficult and we would also have to consider migrating foreign material (e.g. grass awn) as an outside possibility. Dr. Koehler noted that after the animal’s death, nematodes may continue to actively traverse the CNS and therefore be missed in histologic section. One interesting feature of this case was that the pigmented intestinal epithelial cells of P. tenuis were highlighted with Prussian blue, suggesting that these nematodes accumulate iron through ingestion of tissue and red blood cells. We differed from the contributor’s morphologic diagnosis only in severity, though admittedly, this is separate and distinct from clinical effect whereby even mild processes within the brain can have marked effects.
While the periphery of this section does not have many significant changes, it does provide an opportunity to discuss some of the challenges of processing nervous tissues for histologic section as discussed by Dr. Koehler in her preconference. In some cases, autolysis will soften the brain and chattering or tearing of tissue is inevitable. One quick test for autolysis is to examine glial cells in the parenchyma – well-preserved/fixed tissue should have small, round glial cells. The submitted tissue in this case is fixed appropriately, but possibly had minor deviations in temperature, reagent mixture, and/or workflow that led to artifactual separation – the difference may be as small as a few seconds or a few degrees Fahrenheit! We say all of this to emphasize that the production of quality slides is a laborious process and histology technicians all around the world work minor miracles every day to allow us to do the work that we do. We appreciate what the contributor provides here and provide this note as a reference to those starting out on their neuropathology journey.
References:
- Anderson RC. The ecological relationships of meningeal worm and native cervids in North America. Wildl. Dis. 1972; 8:304–310.
- Dobey CL, Grunenwald C, Newman SJ, Muller L, Gerhold RW. Retrospective study of central nervous system lesions and association with Parelaphostrongylus species by histology and specific nested polymerase chain reaction in domestic camelids and wild ungulates. J Vet Diagn Invest. 2014 Nov;26(6):748-54.
- Bak EJ, Jean YH, Woo GH. Eosinophilic encephalomyelitis in horses caused by protostrongylid parasites. J Vet Sci. 2017 Dec 31;18(4):551-554.
- Gardiner, CH, Poynton, SL. An Atlas of Metazoan Parasites in Animal Tissues. (1999).
- Gerhold RW, Keel MK, Arnold K, Hotton D, Beckstead RB. Parelaphostrongylus tenuis-associated meningoencephalitis in a sika deer (Cervus nippon). J Wildl Dis. 2010 Jan;46(1):287-90.
- Lankester, MW. Extrapulmonary lungworms of cervids. In: Samuel, W, Pybus, M, Kocam, A, eds. Parasitic diseases of Wild Mammals. Iowa State University Press, 2001: 228-246.
- Mitchell, KJ, et al. Diagnosis of Parelaphostrongylus spp . infection as a cause of meningomyelitis in calves. Vet. Diagnostic Investig. 2011; 23:1097–1103.
- Southard T, Bender H, Wade SE, Grunenwald C, Gerhold RW. Naturally occurring Parelaphostrongylus tenuis-associated choriomeningitis in a guinea pig with neurologic signs. Vet Pathol. 2013 May;50(3):560-2.
- Tanabe M, Gerhold RW, Beckstead RB, de Lahunta A, Wade SE. Molecular confirmation of Parelaphostrongylus tenuis Infection in a horse with verminous encephalitis. Vet Pathol. 2010 Jul;47(4):759.
- Wünschmann A, Armien AG, Butler E, Schrage M, Stromberg B, Bender JB, Firshman AM, Carstensen M. Necropsy findings in 62 opportunistically collected free-ranging moose (Alces alces) from Minnesota, USA (2003-13). J Wildl Dis. 2015 Jan;51(1):157-65.