Signalment:
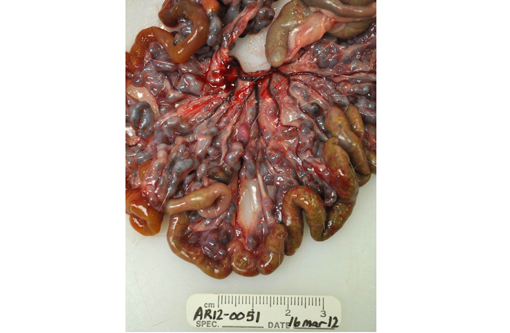
Gross Description:
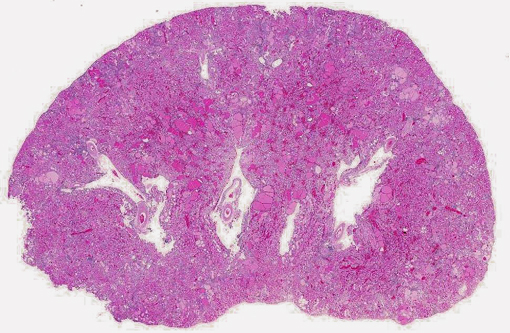
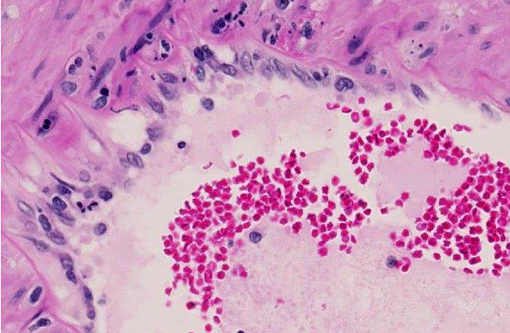
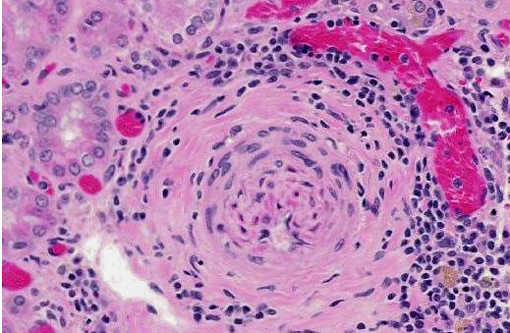
Histopathologic Description:
Morphologic Diagnosis:
1. Glomerulonephropathy, diffuse, chronic, severe with tubular proteinosis and interstitial lymphocytic nephritis and fibrosis (chronic progressive nephropathy).
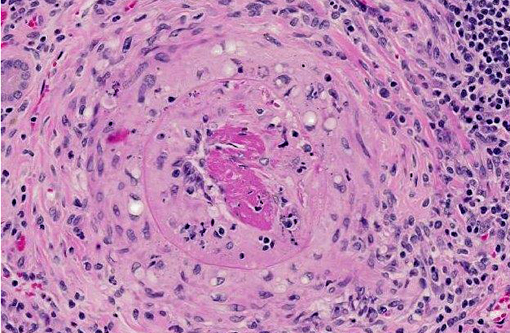
2. Arteritis, segmental, chronic, moderate to severe, lymphohistiocytic with fibrinoid change, kidney (polyarteritis nodosa).
3. Smooth muscle hyperplasia, segmental, chronic, moderate, arterial tunica media, kidney (hypertensive change).
Condition:
Contributor Comment:
Similar to CPN, polyarteritis nodosa is a chronic progressive degenerative disease occurring most frequently in aged Sprague-Dawley rats, spontaneous hypertensive rats, and rats with late-stage chronic nephropathy. At necropsy the mesenteric vessels are often enlarged, tortuous and segmentally thickened, as in this case. Polyarteritis nodosa is characterized histologically by fibrinoid degeneration and thickening of the tunica media of medium-sized arteries with infiltrating mononuclear cells and fewer neutrophils. The lumina may vary in size and have thromboses, some of which may recanalize. Polyarteritis nodosa has also been reported in mice,(7) cats,(1) dogs,(11) pigs,(2,4) cynomolgus macaques(9) and foxes.(6) Although the cause is uncertain, the lymphohistiocytic inflammation suggests it may have an immune-mediated basis.(4,7,9)
The rat is a common animal model of hypertension. Spontaneously hypertensive Wistar rats, Dahl-salt sensitive rats, transgenic mREN2 rats, and rats administered deoxycorticosterone acetate (DOCA) in combination with a high salt diet are the most common models.(3,8) In all of these models, the hypertensive animals have impaired endothelium-dependent relaxation and vascular remodeling which lead to increased vascular resistance and renal damage.(3,8) Severe end-organ damage involving the heart, brain and kidney is only seen in a subset of animals within some models. This rat was transgenic (TGR[mREN2]27), with over-expression of the mouse Ren-2 gene which increases renin activity. Renin catalyzes the conversion of angiotensinogen to angiotensin I, which is then converted to angiotensin II by angiotensin-converting enzyme. Angiotensin II causes vasoconstriction and increased blood pressure. Hypertensive changes are further exacerbated by angiotensin II-induced aldosterone secretion which increases mineralocorticoid levels and sodium reabsorption, thus increasing blood volume osmotically and further increasing blood pressure.(10)
JPC Diagnosis:
1. Kidney, arteries: Arteritis, proliferative and necrotizing, multifocal, severe, with fibrinoid necrosis.
2. Kidney, arteries: Arteriosclerosis, multifocal, moderate.
3. Kidney: Glomerulonephritis, diffuse, with tubular degeneration, necrosis and regeneration, proteinosis, and chronic interstitial nephritis.
Conference Comment:
Conference participants discussed the vessel wall changes observed in this case, noting that hypertension results in several pathologic changes in the walls of small arteries and arterioles, including two types of arteriolosclerosis: hyaline and hyperplastic. Small blood vessels in hyaline arteriolosclerosis exhibit a homogeneous, eosinophilic thickening of the wall and narrowing of the lumen due to endothelial cell damage and the subsequent increased vascular permeability and plasma protein leakage into the vessel wall. Hyperplastic arteriolosclerosis, on the other hand, is characterized by a concentric, laminated thickening of the wall (onion skinning) and narrowing of the lumen which is due to smooth muscle cell hyperplasia and reduplication of basement membranes. Hyperplastic arteriolosclerosis can also be associated with fibrinoid necrosis in the vessel walls.(10)
References:
2. Elling F. Nutritionally induced necrotizing glomerulonephritis and polyarteritis nodosa in pigs. Acta Pathol Microbiol Scand A. 1979;87A(1-6):387-392.
3. Dornas W, Silva ME. Animal models for the study of arterial hypertension. J Biosci. 2011;36(4): 731-737.
4. H+â-¬lie P, Drolet R, Germain MC, Bourgault A. Systemic necrotizing vasculitis and glomerulonephritis in grower pigs in southwestern Quebec. Can Vet J. 1995;36(3):150154.
5. Miller LM, Van Vleet JF, Gal A. Cardiovascular system and lymphatic vessels. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:552.
6. Nordstoga K, Westbye K. Polyarteritis nodosa associated with nosematosis in blue foxes. Acta Pathol Microbiol Scand A. 1976;84(3):291-296.
7. Percy, DH, Barthold SW. Rat. In: Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, Iowa: Blackwell Publishing; 2007:125-206.
8. Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res. 1998;39:77-88.
9. Porter BF, Frost P, Hubbard GB. Polyarteritis Nodosa in a Cynomolgus Macaque (Macaca fascicularis). Vet Pathol. 2003;40(5):570-573.
10. Schoen FJ. Blood Vessels. In: Robbins and Cotran Pathologic Basis of Diseases. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:487-528.
11. Scott-Moncrieff JC, Snyder PW, Glickman LT, Davis EL, Felsburg PJ. Systemic necrotizing vasculitis in nine young beagles. J Am Vet Med Assoc. 1992;201(10):1553-1558.