CASE II: LE-2 (JPC 4120289)
Signalment:
3-year-old neutered male rabbit (Oryctolagus cuniculus forma domestica
History:
Two days after its partner-animal died without premonitory signs, the presented rabbit was vaccinated against myxomatosis and rabbit hemorrhagic disease virus (RHDV). The used vaccines were Nobivax Myxo-RHD® (owner of license: Intervet international) and Filavac VHD K C+V® (owner of license: Filavie). As the animal showed a good state of health, it was placed in a new group of rabbits 3 days after vaccination. On the next day it was found dead without any premonitory signs.
Gross Pathology:
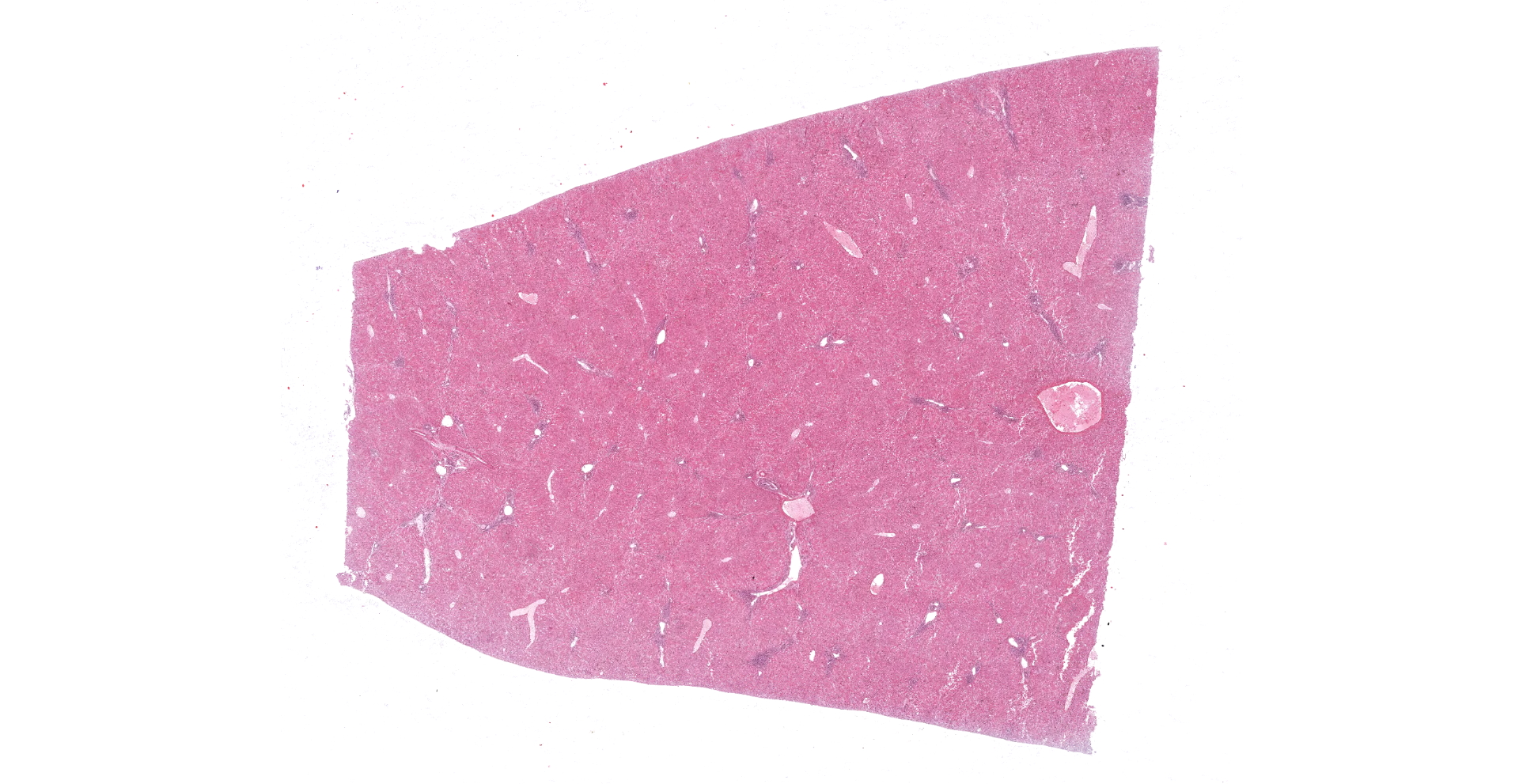
The animal was in a good body condition. The liver showed a diffuse orange to reddish brown coloration and a moderate friable consistence. Multifocal mild to moderate acute hemorrhages were detectable within the lung. Bilateral in the axillary subcutis a lipoma (each 2 cm in diameter) was visible. No further pathomorphological alterations could be observed.
Laboratory Results:
The molecular testing of a pooled sample of liver and lung by real-time PCR to detect rabbit hemorrhagic disease virus (RHDV) was finished with a positive result. The sequence alignment of the PCR-product showed a 97 % homology to RHDV type 2.
The parasitological investigation of the intestine was finished with a negative result.
Microscopic Description:
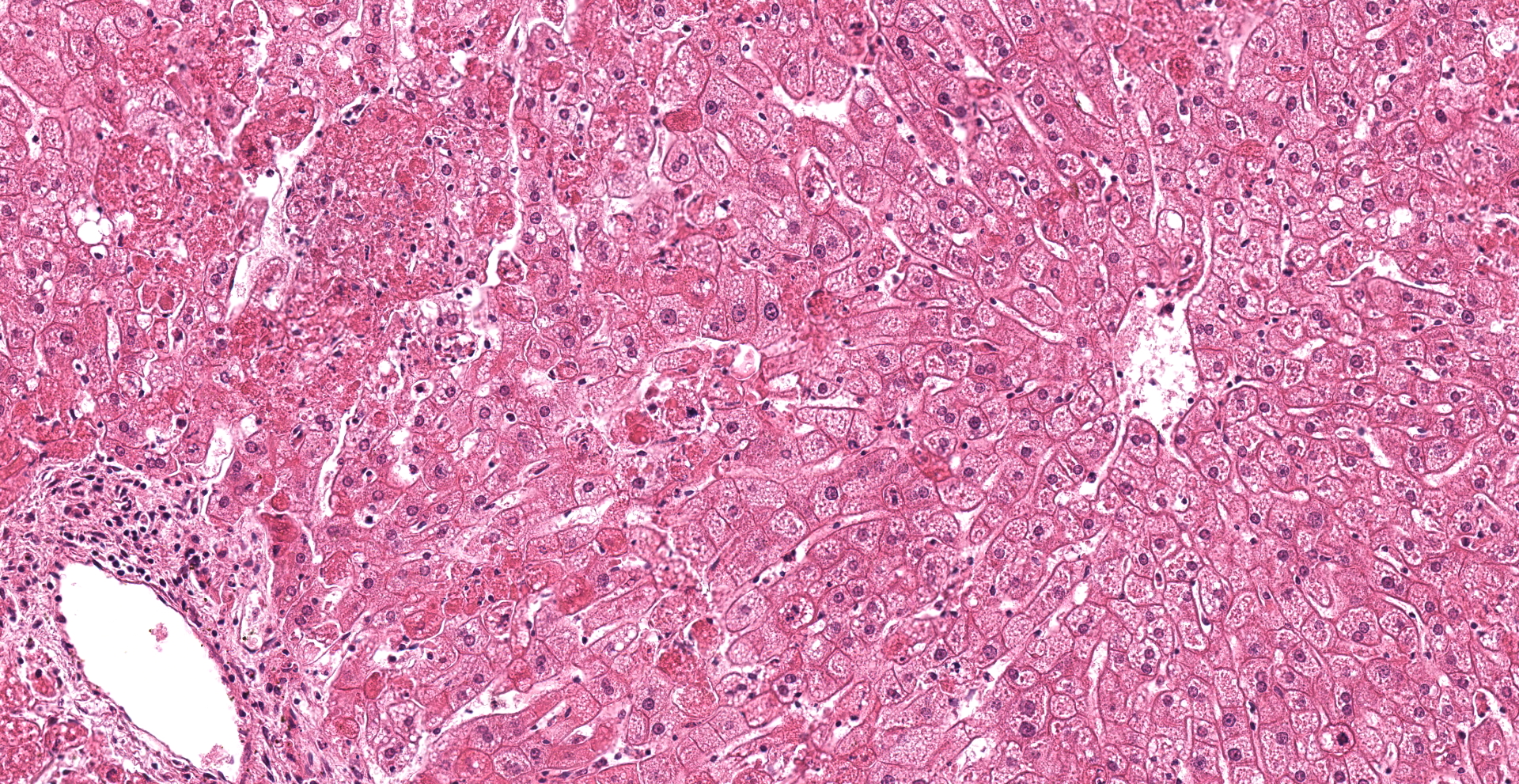
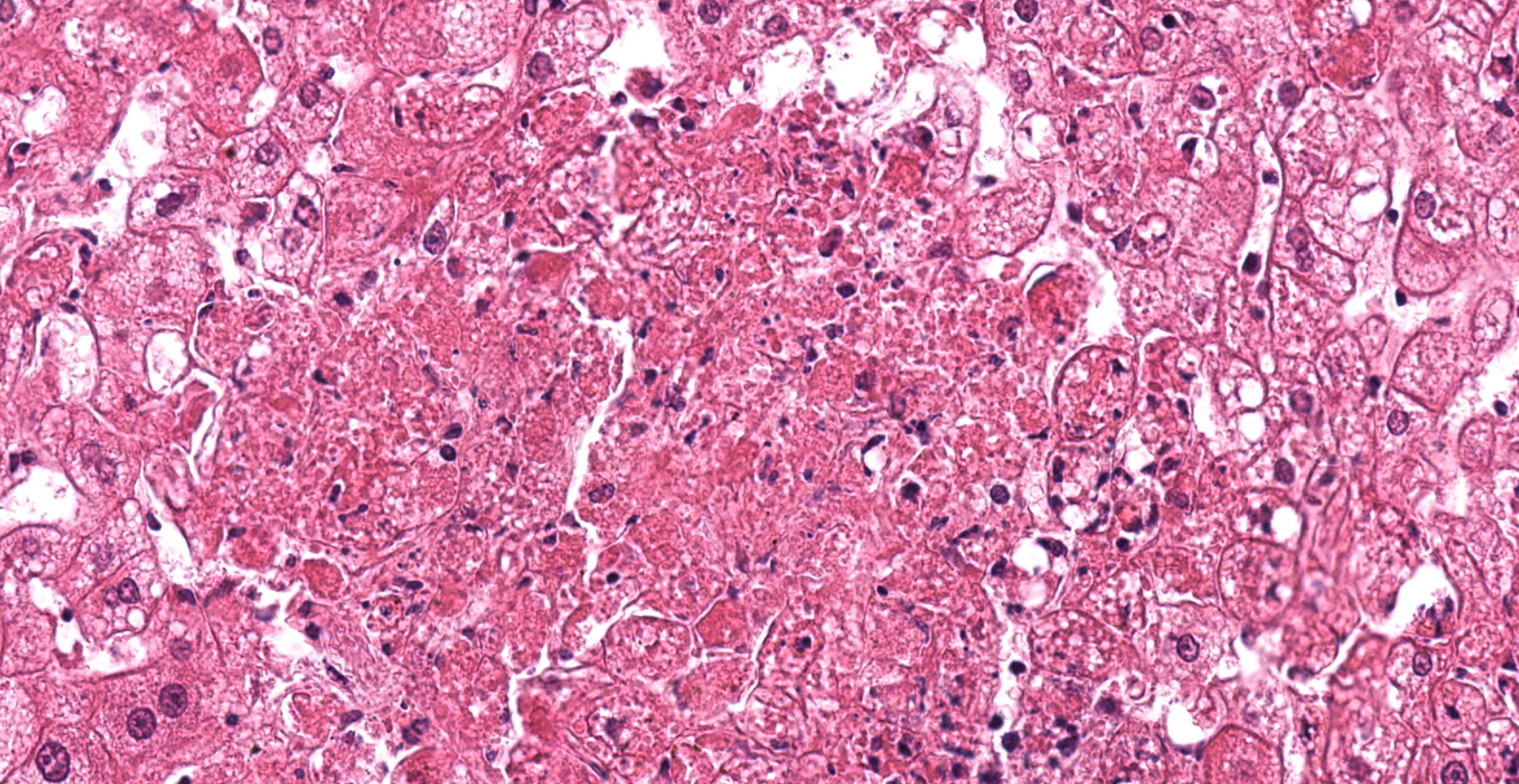
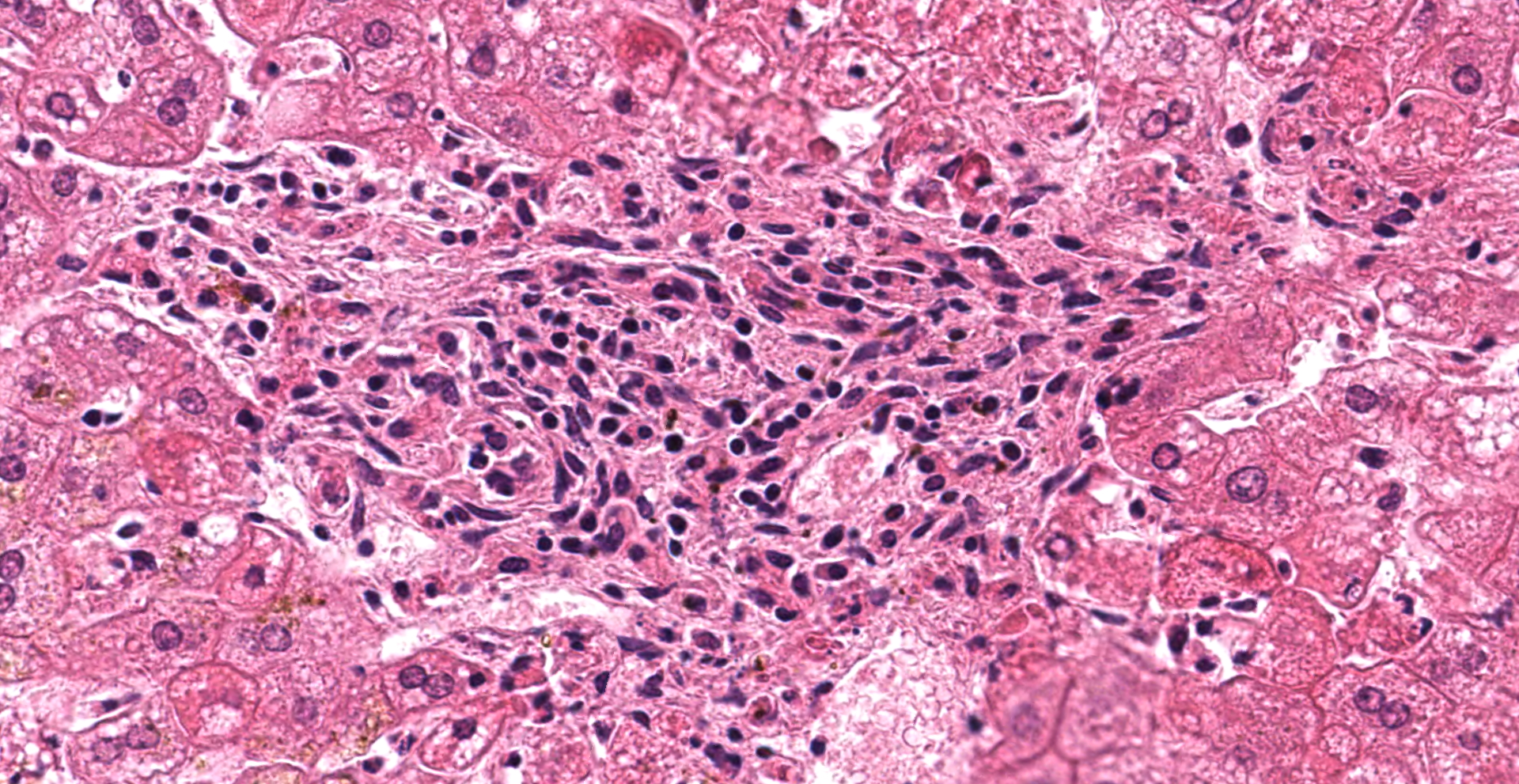
Liver: Multifocal to coalescing a moderate to severe hepatocellular necrosis is detectable, which can predominantly be found in periportal and midzonal areas of the hepatic lobules. The affected hepatocytes are characterized by a hyper-eosinophilic, frequently shrunken and fragmented hepatocellular cytoplasma as well as karyopyknosis and karyolysis. Some necrotic areas show slight hemorrhages and are scattered infiltrated by mostly degenerated neutrophils. Due to dissociation of the damaged hepatocytes, the liver cords are multifocally discontinuous and irregular. Numerous of the remaining hepatocytes are enlarged and show a fine foamy cytoplasma, frequently revealing tiny lipid droplets or sometimes vacuoles. Infrequent, fatty cysts are visible. Binucleated hepatocytes are a common finding. Multifocal the periportal areas show a proliferation of the connective tissue, characterized by a mild to moderate fibroplasia and scattered early proliferation of bile ducts. In these areas a mild to moderate mononuclear portal/periportal infiltration, predominantly characterized by lymphocytes as well as several plasma cells and macrophages can be detected.
In several of the remaining hepatocytes, Kupffer cells and periportal located macrophages, a mild storage of a dark brown coarse-grained pigment can be observed, consistent with hemosiderin (staining by Berlin-Blue: positive).
Contributor's Morphologic Diagnoses:
Liver:
1. Hepatocyte necrosis, acute, multifocal to coalescing, moderate to severe, consistent with the diagnosis of viral hemorrhagic disease of rabbits
2. Proliferation of connective tissue, (peri) portal, multifocal, mild to moderate with mononuclear infiltration, multifocal, mild to moderate
Contributor's Comment:
The rabbit hemorrhagic disease (RHD), also referred as viral hemorrhagic disease (VHD) of rabbits, is an acute and highly contagious viral hepatitis with a mortality rate ranging between 70 % and 100 %. RHD affects both European domestic and wild rabbits (Oryctolagus cuniculus).1,6 First described in China (1984), the disease rapidly spread worldwide and occurs on almost all continents, being endemic in most parts of Europe, Asia, parts of Africa, Australia and New Zealand.1 The causative agent is the rabbit hemorrhagic disease virus (RHDV), a calicivirus belonging to the genus Lagovirus.12,22,25,28,29,33 Like other caliciviruses, it is a small sized (about 35-40 nm in diameter), non-enveloped, icosahedral single-stranded RNA-virus.25,29,36,39
RHDV is mainly transmitted directly by contact to infected animals which shed viral particles in their secretions and excretions (oral, nasal, conjunctival, parenteral), but indirect transmission (fomites-contaminated food, water, bedding, cages, clothing, equipment) as well as a vector-borne transmission (scavenging mammals, birds, blood-feeding insects insects) is described.4,7,9,24,39 In natural infections the fecal-oral route is considered to be the preferential way of transmission.4,24 Virus injected into bovine liver, which was used to mimic RHDV in rabbit carcasses, was still viable after 3 months.14 Due to this high resistance and stability, carcasses of RHDV-infected animals are considered as an important source for viral spreading in the field.14,21,23
The incubation period of RHD ranges between 24 and 72 hours, whereat the symptoms occur suddenly, often without conspicuous signs, and usually with an elevated temperature of up to 40-41.5°C.23 Three clinical courses of the disease can be differentiated: peracute, acute and subacute/mild.23,39 No clinical signs are visible in the peracute form. In contrast, animals with acute infections show unspecific signs as anorexia and apathy, but also neurological symptoms (e.g. excitement, paralysis ophistotonus, ataxia) and occasionally respiratory signs (tracheitis, dyspnea and cyanosis), foamy or bloody nasal discharge, lacrimation, ocular hemorrhage, epistaxis, blood in feces, icteric coloration of the subcutis and skin of the ears, coagulation disorders, anemia and leucocytosis are described.11,16,20,23,39 Subacute/mild forms present similar but milder clinical symptoms and most rabbits survive and develop antibodies against RHDV, which confer protection upon re-infection.23,29
Naturally occurring RHD due to infection with the "classical" RHDV is rarely seen in rabbits less than two months of age and the most studies on experimentally-infected animals were only able to detect viral antigens in rabbits older than 4-weeks.20,30,34,39 The viral dissemination in these young (so-called "resistant") animals is so far unclear, but is possibly due to differences in the leucocyte response to hepatocyte infection between adult and juvenile rabbits. In this context the lymphocytic rather than the heterophilic response is observable in younger animals, which possibly reflects a protective host response to viral antigens on the hepatocyte surface.1,8
In adult rabbits the primary target tissues of RHDV are liver, lung and spleen.2,10,16,25,32 A viral replication in the cytoplasm of hepatocytes is detectable within the first hours post infection, reaching a maximum after 36-48 hours.2,11,15,30 Moreover, RHDV can be observed in macrophages within the red pulp of the spleen and lymph node sinuses as well as within alveolar macrophages of the lung and Kupffer cells of the liver but also in circulating monocytes of unaffected organs such as enteric submucosae, myocardium and thymus.2,16,30,32 Therefore it is supposed, that macrophages are important for spreading the infection.16,32
The most conspicuous lesions at necropsy are mostly found in liver, trachea and lungs.20 The pale yellow or grayish and friable liver can be enlarged and is often characterized by a fine lobular appearance and sometimes interspersed with haemorrhages.5,11,15,16,20,28 Histomorphologically, the characteristic finding is a severe hepatocellular necrosis especially in the peripheral zones of lobules, which is infiltrated by neutrophils (acute necrotic hepatitis).10,11,16,20,30 As also shown in the present case, affected hepatocytes are characterized by hyper-eosinophilia, karyopyknosis and karyorrhexis. The liver cords become discontinuous and irregular as a consequence of hepatocyte dissociation and small, scattered intralobular foci of hemorrhage are present.10,20,30 Further hepatocytic lesions, also observed in the remaining hepatocytes of the present case, are a fine foamy cytoplasma (tiny lipid droplets or sometimes vacuoles) as well as bile pigment and/or iron pigment deposition.10,20,30 In the present case, hepatocellular necrosis can not only be detected in the peripheral but also in the midzonal areas of the lobules. Only few, mostly karyorrhectic neutrophils are visible, whereas several hepatocytes are enlarged and binucleated. Moreover, the (peri-)portal areas show a proliferation of connective tissue, characterized by a mild to moderate fibroplasia and scattered early proliferation of bile ducts, accompanied by mild to moderate mononuclear infiltration of the portal tracts. Similar findings could be made in experimentally RHDV-infected animals killed 7 days post infectionem.10 Therefore the alterations can be interpreted as early signs of regeneration or rather reparation, which is in line with a subacute process and indicates a subacute/mild form of RHD in the present case.
As a characteristic feature of disseminated intravascular coagulation (DIC) in RHD a frequent histopathological finding are microthrombi in small blood vessels especially from liver, spleen and kidney but also in the brain and alveolar capillaries.20,30 Consequential, almost all organs, particularly lungs, heart and kidney are characterized by petechial haemorrhages.10,20,32 Moreover, congestions, especially of the lung, trachea, kidney and spleen, splenomegaly as well as an oedema of the lung and abundant frothy fluid in the trachea are frequent features.5,10,11,15,16,20,28,32
As infrequent findings in RHD profuse bloody effusion in the thoracic and abdominal cavities, poor blood coagulation, icterus, catarrhal gastritis with mucosal erosions and hyperplastic enlargement of mesenteric lymph nodes, karyorrhexis and karyopyknosis in cells of the spleen and other lymphoid tissue (BALT, GALT, thymus, lymph nodes), leading to depletion and lymphopenia are described.5,20,30,39 Few authors report membranous glomerulonephritis, non-suppurative encephalomyelitis, gastritis, adrenocortical necrosis, endometrial congestion and hemorrhages, degeneration of the pancreatic acinar epithelial and islet cells, focal necrosis in the mucosa of the gall bladder, and inflammatory cells and serous exudate filling pulmonary airways and alveoli.39 In pregnant rabbits, fetuses with multi-organic focal hemorrhages were found.5
In summary, a severe necrotising hepatitis, especially in the peripheal zones of lobules as well as a DIC, followed by hemorrhages and congestions, particularly in the lungs, heart and kidney and splenomegalia are the most consistent pathological findings in RHD.2,10,11,16,23,28,32
In 2010, a new lagovirus was identified in France, spreading throughout Europe within a couple of years now reaching Australia.17,18,26,27,31 This new virus showed a capsid protein sequence identity of about 80 per cent with RHDV and was able to cause RHD, also in vaccinated animals.17 Therefore it was termed RHDV type 2 (RHDV2). As also shown in the presented case pathomorpholgical findings in rabbits infected by RHDV2 are consistent with those of RHD caused by the "classical variant" of RHDV.5,17
In contrast to the "classical variant" of RHDV, RHDV2 causes not only RHD also in young rabbits (4 weeks old), but also a RHD-like disease in different hare species, including European brown hares (Lepus europaeus).5,13,18,19,31,37 These special features of RHDV2 strongly suggest, that the virus did not originate from RHDV but rather that it had recently emerged from an unknown source.6
While in experimental infections with the early detected strains of RHDV2 a mortality rate of only 20-30 per cent could be detected, experimental infections of rabbits with two Italian RHDV2-strains isolated in 2014 and 2015 induced at least 80 % mortality rate.6,18 These findings approaches the usual mortality rate of RHDV and is four times higher than that found in the early RHDV2-isolates.6,18
Nowadays, RHDV2 is the dominant virus causing RHD in most European countries, but "classical variants" of RHD still coexists in some areas, also in Germany.6,35 Therefore, it is advisable to vaccinate not only against RHDV2 but also against the "original" RHDV strains.6,35 In the present case this advice was fulfilled by using two different vaccines: Nobivax Myxo-RHD® (owner of license: Intervet international) and Filavac VHD K C+V® (owner of license: Filavie). While the first one (live vaccine, antigens RHD/myxomatosis) is used to protect against myxomatosis and the "original" RHDV-strains, the second contains an inactive strain of RHDV and RHDV2, and is therefore used for the immunization of rabbits against "original" RHDV-strains as well as the new RHDV2-variant.35 Nevertheless the presented animal died following RHDV2-infection. There are no datas given, nor regarding the state of health within the new animal group the rabbit was placed in, nor regarding the cause of death of the partner-animal. A complete immunization by Filavac VHD K C+V® is accomplished not until seven days after vaccination.35 Therefore, there are two possible sources of RHDV2 thinkable in the present case: First, the dead partner-animal and second an infected animal within the new group. In this context it has to be considered, that the histomorphological findings (proliferation of the connective tissue, fibroplasia, early proliferation of bile ducts, mononuclear infiltration of the portal tracts) are in line with a subacute process. Due to these findings, it can be assumed that the RHDV2-infection already occurred before vaccination. Therefore, the peracute death of the partner-animal two days before vaccination can, in all probability, be assigned to a peracute form of RHD and can be considered as the source of RHDV2 in the present case. Most rabbits survive subacute forms of RHD and develop antibodies.23,29 In the present case it is thinkable, that vaccination of an already infected animal worsened the course of disease.
Contributing Institution:
Institute of Pathology, Faculty of Veterinary Medicine, University of Leipzig; An den Tierkliniken 33, 04103 Leipzig, Germany; patho.vetmed.uni-leipzig.de
JPC Diagnosis:
Liver: Hepatitis, necrotizing, acute, multifocal, random, moderate, with hepatocellular vacuolar change.
JPC Comment:
The contributor provides an outstanding and thorough review of the emergence, clinical signs, pathogenesis, and host factors associated with both classical RHDV and RHDV2, two significant causes of mortality in lagomorphs. Since the submission of this case to the WSC in 2019, RHDV2 has been reported in North America, with outbreaks occurring in both Canada and the western United States.3,38
An outbreak of RHDV2 in the southwestern United States was first detected in New Mexico during March 2020. Positive cases were later identified in Texas, Colorado, Arizona, Utah, Nevada, and southwestern California by August 2020. Affected species predominantly included wild lagomorphs such as the black-tailed jackrabbit (Lepus californicus) and desert cottontail rabbit (Sylvilagus audubonii). In addition, RHDV2 was isolated from a domestic rabbit on July 10th, 2020, indicating spillover into the domestic rabbit population. In response, the California Department of Food and Agriculture banned the entrance of rabbits, hares, and any associated products and equipment from any affected state within the previous 12 months. In addition having a negative economic impact, this disease also poses a significant threat to the endangered riparian brush rabbit (Sylvilagus bachmani riparius).1
An additional RHDV2 outbreak in the United States was identified along the northwestern coast of Washington State in July 2019, with affected regions including Orcas Island, San Juan Island, Whidbey Island, and Clallam County located on the Washington State mainland. This outbreak likely extended from a 2018 RHDV2 outbreak along the coastal region of British Columbia, Canada. Affected rabbits included a 2-year-old, 4-H pet, Norwegian dwarf buck, feral domestic rabbits, and 108 of 145 rabbits at rescue organization. Samples of liver and spleen (14 total) were submitted to the USDA-APHIS Foreign Animal Disease Laboratory (FADDL; Plum Island, NY, USA) from the Washington Animal Disease Diagnostic Laboratory (WADDL) for antigen ELISA and reverse transcriptase PCR (RT-PCR), confirming RHDV2 in all submitted samples. Although histologic lesions of rabbits affected in this outbreak were similar to those described in European outbreaks of RHDV2, there were notable differences regarding gross and histologic findings. Icterus has been historically described to be a prominent feature of this entity, which was absent in all cases submitted to the WADDL. Histologically, hepatic lesions in the WADDL cases were randomly distributed, with all lobular zones affected, which contrasts with the clear periportal hepatocellular necrosis described in European cases. The underlying cause of this alternative pattern of distribution is unknown but may be due to differences in cellular tropism, pathogenesis, host factors, or other variables. Therefore, RHDV2 should be considered as a differential in cases of unexpected lagomorph death, including those cases lacking periportal hepatocellular necrosis.38
There was spirited discussion amongst participants in regard to the distribution of hepatic lesions in this case. A significant minority favored a periportal to midzonal distribution, as typically associated with this entity. However, random hepatocellular necrosis has also been reported with this entity, as previously discussed with reference to the 2019 outbreak of RHDV2 in Washington State. In addition, conference participants noted periportal lymphocytic and heterophilic inflammation, which is not a typical finding associated with rabbit hemorrhagic disease. The cause of this inflammation is unclear, though participants suggested Eimeria stiedae, a common apicomplexan parasite of rabbit biliary epithelium, as a differential. However, other features of this entity, such as biliary hyperplasia and the presence of apicomplexan gametocytes, schizonts, and oocysts were not observed.
References:
1. Abrantes J, van der Loo W, Le Pendu J, Esteves PJ. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res. 2012;43:12.
2. Alonso C, Oviedo JM, Martίn-Alonso JM, Dίaz E, Boga JA, Parra F. Programmed cell death in the pathogenesis of rabbit hemorrhagic disease. Arch Virol. 1998;143:321-332.
3. Asgari S, Hardy JR, Sinclair RG, Cooke BD. Field evidence for mechanical transmission of rabbit haemorrhagic disease virus (RHDV) by flies (Diptera: Calliphoridae) among wild rabbits in Australia. Virus Res. 1998;54:123-132.
4. Asin J, Nyaoke AC, Moore JD, et al. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J Vet Diagn Invest. 2021;33(4):728-731.
5. Camarda A, Pugliese N, Cavadini P, et al. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res Vet Sci. 2014;97:642?645.
6. Capucci L, Cavadini P, Schiavitto M, Lombardi G, Lavazza A. Increased pathogenicity in rabbit haemorrhagic disease virus type 2 (RHDV2). Vet Rec. 2017; doi: 10.1136/vr.104132
7. Cooke BD. Rabbit haemorrhagic disease: field epidemiology and the management of wild rabbit populations. Rev Sci Tech. 2002;21:347-358.
8. Ferreira PG, Costa-E-Silva A, Oliveira MJR, Monteiro E, Aguas AP. Leukocyte-Hepatocyte interaction in calicivirus infection: Differences between rabbits that are resistant or susceptible to rabbit haemorrhagic disease (RHD). Vet Immunol Immunopath. 2005;103:217-221.
9. Frölich K, Klima F, Dedek J. Antibodies against rabbit hemorrhagic disease virus in free-ranging red foxes from Germany. J Wildl Dis. 1998;34:436-442.
10. Fuchs A, Weissenbock H. Comparative histopathological study of rabbit haemorrhagic disease (RHD) and European brown hare syndrome. J Comp Path. 1992;107:103-113.
11. Gelmetti D, Grieco V, Rossi C, Capucci L, Lavazza A. Detection of rabbit haemorrhagic disease virus (RHDV) by in situ hybridisation with a digoxigenin labelled RNA probe. J Virol Methods. 1998;72:219-226.
12. Green KY, Ando T, Balayan MS, et al. Taxonomy of the Caliciviruses. J infect Disease. 2000;181(Suppl 2):322-330.
13. Hall RN, Peacock DE, Kovaliski J, et al. Detection of RHDV2 in European brown hares (Lepus europaeus) in Australia. Vet Rec. 2017;180:121
14. Henning J, Meers J, Davies PR, Morris RS. Survival of rabbit haemorrhagic disease virus (RHDV) in the environment. Epidemiol Infect. 2005;133:719-730.
15. Jung JY, Lee BJ, Tai JH, Park JH, Lee YS. Apoptosis in rabbit haemorrhagic disease. J Comp Pathol. 2000;123:135-140.
16. Kimura T, Mitsui I, Okada Y, et al. Distribution of rabbit haemorrhagic disease virus RNA in experimentally infected rabbits. J Comp Pathol. 2001;124:134-141.
17. Le Gall-Reculé G, Zwingelstein F, Boucher S, et al. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec. 2011;168:137?138.
18. Le Gall-Reculé G, Lavazza A, Marchandeu S, et al. Emergence of a new lagovirus related to Rabbit Haemorrhagic Disease Virus. Vet Res. 2013;44:81?94.
19. Le Gall-Reculé G, Evelyne Lemaitre E, Bertagnoli S, et al. Large-scale lagovirus disease outbreaks in European brown hares (Lepus europaeus) in France caused by RHDV2 strains spatially shared with rabbits (Oryctolagus cuniculus). Vet Res. 2017;48:70 doi:10.1186/s13567-017-0473-y
20. Marcato PS, Benazzi C, Vecchi G, et al. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991;10:371-392.
21. McColl KA, Morrissy CJ, Collins BJ, Westbury HA. Persistence of rabbit haemorrhagic disease virus in decomposing rabbit carcases. Aust Vet J. 2002;80:298-299.
22. Meyers G, Wirblich C, Thiel HJ. Rabbit hemorrhagic disease virus?molecular cloning and nucleotide sequencing of a calicivirus genome. Virology 1991;184:664-676.
23. Mitro S, Krauss H. Rabbit hemorrhagic disease: a review with special reference to its epizootiology. Eur J Epidemiol. 1993;9:70-78.
24. Morisse JP, Le Gall G, Boilletot E. Hepatitis of viral origin in Leporidae: Introduction and aetiobogical hypotheses. Rev sci tech Off int Epiz. 1991;10:283-295.
25. Moussa A, Chasey D, Lavazza A, et al. Haemorrhagic disease of lagomorphs: evidence for a calicivirus. Vet Microbiol. 1992;33:375-381.
26. Mutze G, De Preu N, Mooney T, et al. Substantial numerical decline in South Australian rabbit populations following the detection of rabbit haemorrhagic disease virus 2. Vet Rec. 2018;182:574; doi:10.1136/vr.104734
27. Neimanis AS, Ahola H, Zohari S, et al. Arrival of rabbit haemorrhagic disease virus 2 to northern Europe: Emergence and outbreaks in wild and domestic rabbits (Oryctolagus cuniculus) in Sweden. Transbound Emerg Dis. 2018;65:213?220.
28. Ohlinger VF, Haas B, Meyers G, Weiland F, Thiel HJ. Identification and characterization of the virus causing rabbit hemorrhagic disease. J Virol. 1990;64:3331-3336.
29. Parra F, Prieto M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J Virol. 1990;64:4013-4015.
30. Prieto JM, Fernandez F, Alvarez V, et al. Immunohistochemical localisation of rabbit haemorrhagic disease virus vp-60 antigen in early infection of young and adult rabbits. Res Vet Sci. 2000;68:181-187.
31. Puggioni G., Cavadini P, Maestrale C. The new French 2010 Rabbit Hemorrhagic Disease Virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet Res. 2013;44:96?103.
32. Ramiro-Ibáñez F, Martín-Alonso JM, García Palencia P, Parra F, Alonso C: Macrophage tropism of rabbit hemorrhagic disease virus is associated with vascular pathology. Virus Res. 1999;60:21-28.
33. Rodak L, Smid B, Valicek L, et al. Enzymelinked immunosorbent assay of antibodies to rabbit haemorrhagic disease virus and determination of its major structural proteins. J Gen Virol. 1990;71:1075-1080.
34. Shien JH, Shieh HK, Lee LH. Experimental infections of rabbits with rabbit haemorrhagic disease virus monitored by polymerase chain reaction. Res Vet Sci. 2000;68:255-259.
35. Ständige Impfkommission Veterinärmedizin (StIKo Vet) am Friedrich-Loeffler-Institut Bundesforschungsinstitut für Tiergesundheit, Arbeitskreis kleine Haustiere (Hartmann K, Kohn B, Moritz A et al.). Hinweis auf neue RHDV-2-Impfstoffe. Greifswald-Insel Riems, CA: StiKoVet; 2017
36. Valiček L, Smid B, Rodak L, Kudrna J. Electron and immunoelectron microscopy of rabbit haemorrhagic disease virus (RHDV). Arch Virol. 1990;112:271-275.
37. Velarde R, Cavadini P, Neimanis A. Spillover events of infection of brown hares (Lepus europaeus) with rabbit haemorrhagic disease typ 2 virus (RHDV2) caused sporadic cases of european brown hare syndrome-like disease in Italy and Spain. Transbound Emerg Dis. 2017;64:1750-1761.
38. Williams LBA, Edmonds SE, Kerr SR, Broughton-Neiswanger LE, Snekvik KR. Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States. J Vet Diagn Invest. 2021;33(4):732-735.
39. Xu ZJ, Chen WX. Viral haemorrhagic disease in rabbits: a review. Vet Res Commun. 1989;13:205-212.