Signalment:
Gross Description:
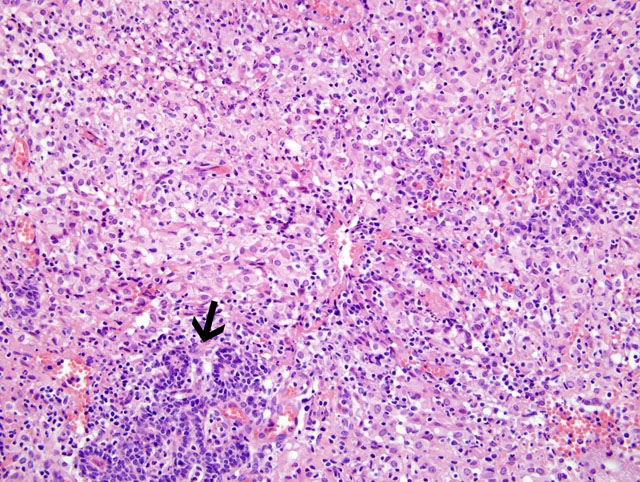
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The guinea pig in this case was experimentally infected via aerosolization with Mycobacterium tuberculosis to evaluate pulmonary tuberculosis. Diagnosis of tuberculosis in the mammary gland was an incidental finding. To the contributors knowledge, tuberculosis mastitis has never been reported in an experimentally infected guinea pig. In human patients, involvement of the mammary gland is a rare manifestation of the disease.(2) Clinically, tuberculoid granulomas of the mammary gland are unilateral and may mimic breast cancer and/or breast abscesses which are all managed differently making accurate diagnosis crucial.(3,9) The reliable diagnostic tests include bacteriologic culture, histopathology, and guinea pig inoculation.(6)
Tuberculosis mastitis (TM) in humans can occur as primary or secondary disease. Secondary involvement of the mammary gland is more common than primary infection of the mammary gland.(6) Common routes of infection include the lymphatic route, hematogenous route or from direct spread from local organs. The direct form of spread in humans may occur from an infected rib, cartilage, or joint.(6)
In humans, there are three forms of mammary tuberculosis: nodular, diffuse, and sclerosing. The nodular form is the most common presentation in women and is typically slow growing and often develops a caseating center.(7) The sclerosing form develops excess fibrous connective tissue and is often the most difficult to differentiate from mammary gland carcinoma.(7)
JPC Diagnosis:
Conference Comment:
Susceptibility to tuberculosis and organ system affected varies greatly among domesticated animal species. The hallmark lesion of tuberculosis is the granuloma. These granulomas may be in different organs with slightly different histologic appearances based on the route of entry and species affected. Below is a brief list of animal species, susceptibility, and organ systems affected by tuberculosis.
| Species | Susceptibility | Organ System Affected |
| Cat | More susceptible to M. bovis | Gastrointestinal disease ingestion of contaminated wildlife or milk |
| Dog | Susceptible to M. bovis and M. tuberculosis | Respiratory form |
| Cattle | More susceptible to M. bovis | Respiratory and gastrointestinal forms calcification can also occur |
| Pigs | Susceptible to both M. bovis and M. tuberculosis | Systemic infection |
| Small ruminants | Rare cases | Similar to cattle when infected |
| Horses | Rare; usually M. bovis | Gastrointestinal; also see lesions in retropharyngeal and mesenteric lymph nodes |
| Non-Human Primates | Very susceptible to M. tuberculosis | Infected humans transmit respiratory form to NHPs |
| Birds (Psittacines) | Only birds to get tuberculosis; get both M. tuberculosis and M. bovis | Respiratory form - transmitted from humans |
References:
2. Bani- Hani KE, Yaghan RJ, Matalka II, Mazahreh TS: Tuberculous mastitis: a disease not to be forgotten. International Journal of Tuberculosis Lung Disease 9(8):920-925, 2005
3. Bedi US, Bedi RS: Bilateral breast tuberculosis. The Indian Journal of Tuberculosis 215-217, 2001
4. Caswell JK, Williams KJ: Respiratory system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 606-610. Elsevier Limited, Philadelphia, PA, 2007
5. Gupta UD, Katoch VM: Animal models of tuberculosis. Tuberculosis 85:277-293, 2005
6. Hale JA, Peters GN, Cheek JH: Tuberculosis of the breast: rare but still extant. The American Journal of Surgery 150:620-624
7. Hamit HF, Ragsdale TH: Mammary tuberculosis. Journal of the Royal Society of Medicine 75:764-765, 1982
8. McMurray DN: Guinea pig model of tuberculosis. In: Tuberculosis: Pathogenesis, Prevention, and Control, ed. Broom BR, pp. 135-147. ASM Press. Washington, DC, 1994
9. Mufide AN, Saglam L, Polat P, Erdogan F, Albayrak Y, Povoski SP: Mammary tuberculosis- importance of recognition and differentiation from that of a breast malignancy: report of three cases and review of the literature. World Journal of Surgical Oncology 5:67-73, 2007