CASE I: Case 2 (JPC 4118311)
Signalment:
Approximately 5 year-old, intact, female, Indian-origin, rhesus macaque (Macaca mulatta)
History:
This macaque was humanely euthanized after reaching experimental endpoint criteria 8 days following intramuscular challenge with a viral agent.
Gross Pathology:
Body as a whole: Good body condition with moderate dehydration
Liver: Severe, subacute, diffuse hepatic necrosis and lipidosis with severe hepatomegaly
Spleen: Moderate, subacute, diffuse necrotizing splenomegaly (fibrinoid necrosis)
Kidneys: Severe, subacute, diffuse renomegaly (congestion and edema)
Lung: Mild, acute, multifocal hemorrhage
Tracheobronchial lymph nodes: Moderate, subacute, diffuse lymphadenopathy
Peripheral and mesenteric lymph nodes: Moderate, subacute, diffuse lymphadenopathy
Adrenal glands: Subacute, moderate, diffuse congestion
Skin covering the chest: Subacute, locally extensive, mild petechial hemorrhage
Dorsal skin of the neck: Subacute, focal, mild ecchymotic hemorrhage
Stomach: Mild, acute gas distention
Laboratory Results: See Tables 1 and 2
|
Table 1 |
|||||
|
Pre- & Post- Challenge Day |
WBC |
Neutrophils x103/µL |
Lymphocytes x103/µL |
Monocytes x103/µL |
Platelets x109/µL |
|
-14 |
6.65 |
3.31 |
2.78 |
0.47 |
311 |
|
-7 |
6.14 |
2.97 |
2.62 |
0.44 |
328 |
|
0 |
6.07 |
2.84 |
2.75 |
0.42 |
371 |
|
3 |
6.60 |
5.24 |
1.12 |
0.24 |
313 |
|
6 |
5.22 |
4.08 |
0.98 |
0.16 |
252 |
|
8 |
8.90 |
3.36 |
4.63 |
0.56 |
379 |
|
Normal Range |
6.4 |
3.5 |
1.6 |
0 |
313 |
|
10.2 |
5.8 |
5.1 |
0.4 |
475 |
|
|
Table 2 |
|||||||
|
Pre- & Post- Challenge Day |
Albumin |
Total Protein |
ALT |
AST |
GGT U/L |
BUN |
Creatinine |
|
-14 |
3.5 |
7.0 |
40 |
28 |
53 |
22 |
0.6 |
|
-7 |
3.5 |
6.5 |
52 |
22 |
52 |
16 |
0.6 |
|
0 |
3.6 |
7.3 |
61 |
33 |
58 |
19 |
0.7 |
|
3 |
3.8 |
6.7 |
68 |
37 |
55 |
21 |
0.9 |
|
6 |
3.4 |
6.2 |
443 |
1,098 |
219 |
- |
1.1 |
|
8 |
2.5 |
5.2 |
1,244 |
ND |
321 |
34 |
3.8 |
|
Normal Range |
3.2 |
5.9 |
26 |
24 |
48 |
16 |
0.8 |
|
4.1 |
7.9 |
52 |
38 |
76 |
22 |
1.2 |
|
Ultrastructural Description:
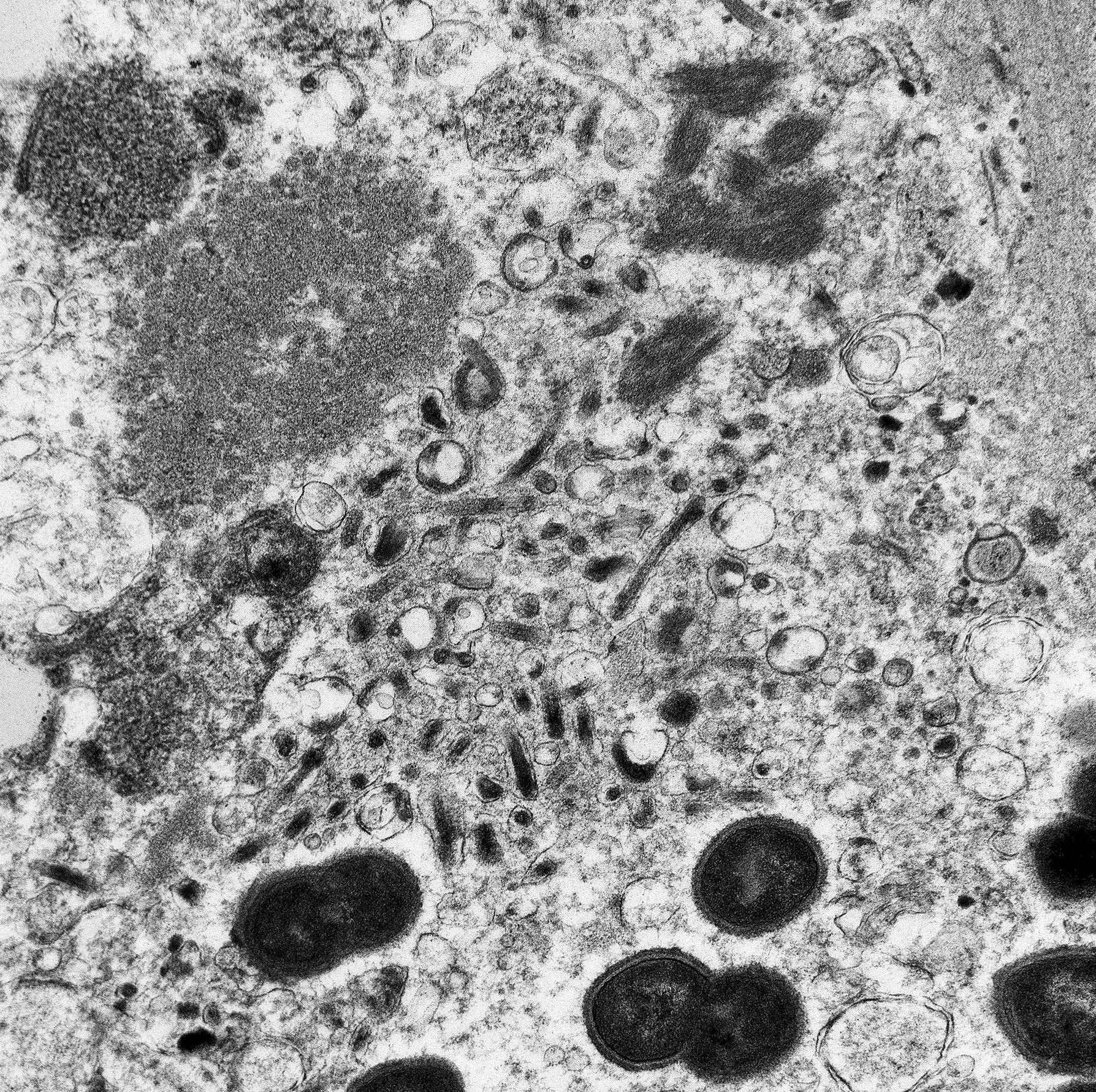
The image contains degenerate organellar cellular debris, variably electron-dense amorphous material (protein), two degenerate cells, approximately 25 single-cell organisms, and myriad filamentous viral particles. The electron-dense, single-cell organisms are round, measure 0.8-1 micron, lack a nucleus, and have a prominent bi-layer cell membrane that is electron lucent centrally. These organisms are sometimes arranged in pairs (diplococci). The filamentous viral particles measure approximately 80 nm in diameter and, within the image, up to 800 nm in length. The viral nucleocapsids are electron-dense with a striated core and surrounded by a viral envelope. The free ends of occasional virus particles fold back onto themselves forming a hook- or loop-like structure multifocally. The two partially-intact cells within the image are polygonal, with indistinct, organellar and cellular membranes. The nuclei in these cells are markedly swollen and moderately electron dense with fragmented, dispersed, electron-dense chromatin. Viral particles are present adjacent to the nuclear membrane, but it cannot be definitively discerned if these are intracytoplasmic viral particles. A medium electron-dense, 1.5x1 micron, aggregate of proteinaceous material (viral nucleocapsids) formed by 20-25 nm tightly-arranged tubules is present in the upper left-hand side of the image (viral inclusion body). Adjacent to this inclusion body are approximately ten, 300-500 nm, elongated clusters of electron-dense material composed of thousands of compact filaments (fibrin).
Contributor's Morphologic Diagnoses:
Tissue: Severe, diffuse, fibrinous necrosis with intralesional myriad filamentous viral particles and multifocal diplococcal bacteria
Contributor's Comment:
The tissue composing the TEM images cannot be discerned. The images are from the spleen of a five-year-old, intact, female macaque inoculated with Marburg virus (MARV) intramuscularly 8 days prior to euthanasia. Histologically the normal architecture of the spleen was effaced by necrosis and bacterial colonies were numerous. Bacterial emboli were also seen in the inoculation site musculature, peripheral and visceral lymph nodes, liver, stomach, small intestine, adrenal glands, bladder, bone marrow, and brain. Bacteria commonly recognized to form diplococci include Gram positive cocci: Streptococcus pneumoniae and Enterococcus spp.; and Gram negative cocci: Neisseria spp., Moraxella catarrhalis, and Acinetobacter spp. The bacterial septicemia observed in this macaque histopathologically is thought to be peracute, as there is no clinical pathological evidence of a response to the bacteria in blood samples collected immediately prior to necropsy or in the tissues collected at necropsy. The bacteria seen in this case are thought to be Streptococcus pneumoniae but additional diagnostics were not performed to identify the bacteria in this case.
The gross, clinical pathologic, and histopathologic lesions seen in this macaque are consistent with those seen in the macaque model of MARV disease.5,8 Pathogenic filoviruses include Ebola virus (Zaire ebolavirus, Sudan ebolavirus, Tai Forest ebolavirus, Bundibugyo ebolavirus) and Marburg virus.7 Reston virus (Reston ebolavirus) is only known to cause disease in primates and pigs; seropositivity alone has been observed in humans following occupational exposure.7 Following exposure, MARV infects, replicates, and disseminates throughout the body in monocytes and macrophages.1 The marked elevations in ALT, AST, and GGT seen in this macaque reflect direct infection and destruction of hepatocytes by MARV.8 The azotemia observed clinically is pre-renal though terminal thrombosis of the renal vasculature due to the coagulopathy induced by MARV is commonly seen.11 Hypoproteinemia is almost universally seen in MARV-infected macaques and thought to be secondary to both hepatic dysfunction and protein loss secondary to increased vascular permeability. Lymphocytes are not infected by MARV but a poorly characterized, "bystander" lymphocytolysis is thought to occur after the onset of infection.4 Widespread lymphoid depletion and necrosis were observed in this macaque terminally, consistent with the modest lymphoid response observed clinicopathologically despite fulminant MARV infection.
Contributing Institution:
Pathology Department
NIH/NIAID
Integrated Research Facility
https://www.niaid.nih.gov/about/integrated-research-facility
JPC Diagnosis:
Undetermined tissue: Cellular degeneration, severe, with intracytoplasmic filoviral particles and cocci.
JPC Comment:
Viral hemorrhagic fever (VHF) in humans is a syndrome characterized by acute fever with systemic involvement and generalized hemorrhage may occur in severe cases. Viral families associated with VHF include genera of Arenaviriade (e.g. Lassa virus), Bunyaviridae (e.g. Crimea-Congo hemorrhagic fever, Rift Valley fever, and Hantavirus), Flaviviridae (e.g. Dengue hemorrhagic fever and yellow fever), and Filoviridae as previously described by the contributor. Although HF viruses are diverse in morphology and size, all are single-stranded RNA viruses with a lipid envelope, which makes them vulnerable to detergents, low pH environments, and household bleach. However, these viruses are stable at a neutral pH, especially in the presence of protein. For example, Ebola virus was cultured from desiccated blood found in syringes stored at room temperature for approximately a month during a Central African outbreak in 1995. All HF viruses, with the exception of dengue viruses, are biosafety level (BSL) 3 or 4 agents due to their tendencies to remain stable and infectious as fine particle aerosols and their ability to produce disease with high morbidity and mortality; both Marburg and Ebola virus are BSL-4 agents.6
Ebola and Marburg virus particles are very similar. Both contain a 19-kb, single, negative-stranded, linear genome encoding seven structural proteins. Three of these proteins, GP, VP24, and VP40, are associated with the membrane. GP is the effector protein for binding and membrane fusion. Interestingly, the GP gene is distinctly different between the Ebola and Marburg viruses. The Marburg virus GP gene encodes a single product (GP) in a conventional open reading frame whereas Ebola virus encode the GP in two open reading frames that are expressed through transcriptional editing. VP 40 is a matrix protein and is responsible for the formation of the particle's filamentous appearance. VP24 may have a role in viral assembly and budding.6
HF viruses exhibit a stereotypical pathogenesis in both humans and non-human primates (NHPs). Following initial infection, the virus spreads to the regional lymph nodes, liver, and spleen where tissue macrophages and dendritic cells are infected, which in turn release cytokines that recruit additional target cells to the site of infection. Although lymphocytes are not directly targeted by the virus, marked lymphocytolysis appears to be the most consistent pathological finding amongst HF viral infections of humans and NHPs, with the exception of hantaviruses. The underlying cause of lymphoid depletion is likely multifactorial, including second order effects from dendritic cell infection and the release of soluble factors from virus infected monocytes and macrophages, resulting in decreased lymphocyte survival signals and upregulation of proapoptitic proteins such as Fas and tumor necrosis factor-related apoptosis virus inducing ligand (TRAIL), respectively. Coagulation anomalies vary in regard to both mechanism and severity between the etiologies. For example, Ebola causes overexpression of tissue factor (factor III), resulting in activation of the extrinsic coagulation pathway and the formation intravascular fibrin thrombi. Coagulation and hemodynamic disturbances are common features of HFVs, largely due to hepatocyte and adrenal cortical cell infection. Hepatocellular infection results in impaired synthesis of both coagulation factors and albumin, predisposing to coagulopathy and decreased oncotic pressure, contributing toward both hypotension and edema. Infection of adrenal cortical cells results in impaired steroid production, resulting in hypotension and sodium loss with hypovolemia.6
Most VHF infections in both humans and NHPs are associated with cutaneous flushing or macular rashes with varying characteristics (though with significant crossover) depending on the agent. More than 50% of humans and macaques infected with Marburg virus and Ebola virus develop nonpruritic petechial dermal rashes on the axillae and groin, forehead, and chest. Arenavirus HF in humans and NHPs is associated with flushed erythematous rashes on the face and thorax, though axillary and oral petechiae are also commonly observed in human cases.6
Histologically, fibrin and fibrinocellular thrombi in numerous tissues and deposition of fibrin in the splenic red pulp and marginal zones are common findings in infected cynomolgus and rhesus macaques.6
As demonstrated by transmission electronic microscopy (TEM) in this case, filoviruses are characterized by their unique filamentous appearance for which they are named (filum; Latin for "thread"). Marburg virus was the first of the filoviruses to be discovered, following three geographically distant but simultaneous VHF outbreaks in European laboratory workers in 1967. The majority of scientific and media attention focused on the most severely affected laboratory, which was in Marburg, West Germany. Infections also occurred in laboratories in Frankfort, West Germany and Belgrade, Yugoslavia.6,10 All primary cases had either direct contact or contact with tissues derived from African green monkeys imported from a single Ugandan primate exporter.9 Secondary transmission to both medical personnel and family members was also reported. Thirty-one patients were infected and seven died (23% mortality). Since 1967, there have been at least 13 additional reports of Marburg virus, most of which manifested as sporadic, isolated, and usually fatal cases in residents and travelers in southeast Africa. However, larger outbreaks such as a 2004-2005 outbreak in Angola that resulted in 200 deaths with a 90% mortality rate have also been reported.6
Notably, Ebola and Marburg virus are both reported to persist in immune-privileged tissues of survivors, including the aqueous humor, central nervous system, and testicles. Sexual transmission of both viruses has been reported. In one case, Ebola virus RNA was detected by reverse transcription PCR in semen 531 days following onset of symptoms.2
Variants of Marburg virus have been isolated from the Egyptian fruit bat (Rousettus aegypticus), which appears to be the natural reservoir, with seasonal circulation changes of Marburg virus in this species being shown to correlate with human infection. Experimentally infected R. aegypticus have been found to orally shed Marburg virus despite showing no clinical signs. Marburg virus has also been identified in the urban-dwelling straw colored bat (Eidolon helvum). In addition to fruit bats, insectivorous bats such as Miniopterus inflatus and Rhinolophus eloquens have also been implicated.3
Interestingly, a third genus of Filoviridae was identified in 2011. Cuevavirus consists of a single species, Lloviu cuevavirus, and was identified following a series of bat die-offs on the Iberian Peninsula. Numerous carcasses of Schreiber's bats (Miniopterus schreibersii) and greater mouse-eared bats (Myotis myotis) were obtained from the Lloviu Cave in northern Spain, from which the virus is named. All the dead M. schreibersii were positive for the virus whereas none was detected in healthy M. schreibersii taken from the same location. Although no gross lesions were noted, histopathological signs of viral pneumonia were present.3
Participants were unable to definitively identify the cell in the submitted TEM image; however, the vast majority suspected the cell to be a macrophage given the pathogenesis of Marburg virus, in addition to the presence of intracellular cocci and the absence of distinguishing features of other cell lines, such as tight junctions with epithelial cells.
References:
1. Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, et al.: Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis 2003:188(11):1630-1638.
2. Den Boon S, Marston BJ, Nyenswah TG, Jambai A, Barry M, Keita S, et al. Ebola Virus Infection Associated with Transmission from Survivors. Emerg Infect Dis. 2019 Feb;25(2):249-255.
3. Emanuel J, Marzi A, Feldmann H. Filoviruses: Ecology, Molecular Biology, and Evolution. Adv Virus Res. 2018;100:189-221.
4. Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB: Apoptosis Induced In Vitro and In Vivo During Infection by Ebola and Marburg Viruses. Laboratory Investigation 2000:80:171.
5. Hensley LE, Alves DA, Geisbert JB, Fritz EA, Reed C, Larsen T, et al.: Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis 2011:204 Suppl 3:S1021-1031.
6. Jahrling PB, Marty AM, Geisbert TW. Viral hemorrhagic fevers. In: Dembek ZF, ed. Medical Aspects of Biological Warfare. Washington, DC: Borden Institute, Walter Reed Army Medical Center, 2007; 271-294.
7. Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brister JR, et al.: Virus nomenclature below the species level: a standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch Virol 2013:158(1):301-311.
8. Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR: Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 2015:235(2):153-174.
9. Miranda MEG, Miranda NLJ: Reston ebolavirus in Humans and Animals in the Philippines: A Review. The Journal of Infectious Diseases 2011:204(suppl_3):S757-S760.
10. Ristanović ES, Koko?kov NS, Crozier I, Kuhn JH, Gligić AS. A Forgotten Episode of Marburg Virus Disease: Belgrade, Yugoslavia, 1967. Microbiol Mol Biol Rev. 2020;84(2):e00095-19. Published 2020 May 13.
11. van Paassen J, Bauer MP, Arbous MS, Visser LG, Schmidt-Chanasit J, Schilling S, et al.: Acute liver failure, multiorgan failure, cerebral oedema, and activation of proangiogenic and antiangiogenic factors in a case of Marburg haemorrhagic fever. Lancet Infect Dis 2012:12(8):635-642.