CASE 1: 11240-12 (4032246-00)
Signalment: 1.5-year-old female St. Bernard dog (Canis lupus familiaris)
History: The owner had taken 3 dogs to a show two weeks previously and they subsequently developed clinical signs of coughing. All dogs (all related St. Bernards) were then treated with trimethoprim sulfa at an unknown dose, over a ten-day period. This dog presented with seizures and hypoglycemia. There was severe anemia, thrombocytopenia, and dry eye. Two other littermates with similar clinical signs and similar treatment were hospitalized, treated symptomatically and eventually recovered. Drug treatment was withdrawn from the other dogs one day following this dog's necropsy.
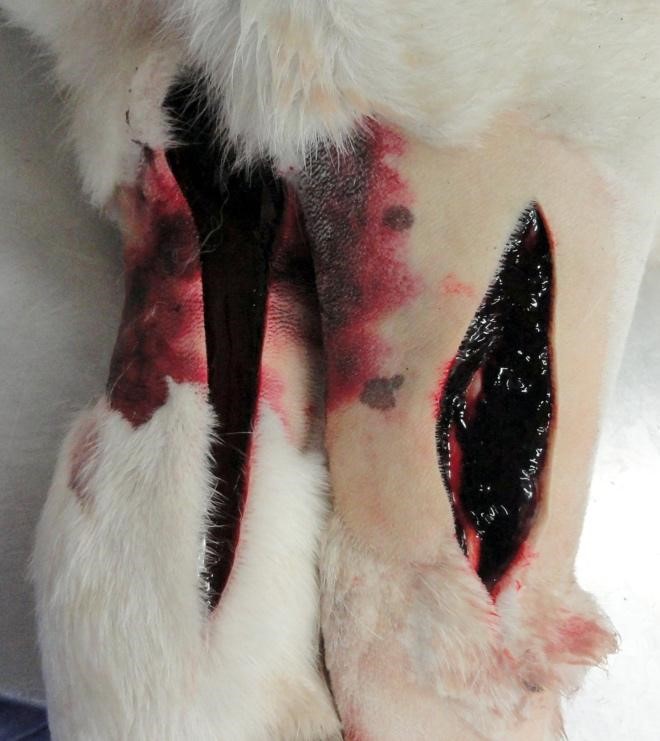
Gross Pathology: This young adult dog had body weight 71.8 kg. The dog was moderately autolyzed. Fecal material on the perineal hair had a red hue. Beginning in the mid-jejunum, intestinal content was dry and consisted of blood. The lower legs were swollen with what was believed to be pitting edema. This was found to be a result of 1-3 cm thick subcutaneous hemorrhage.
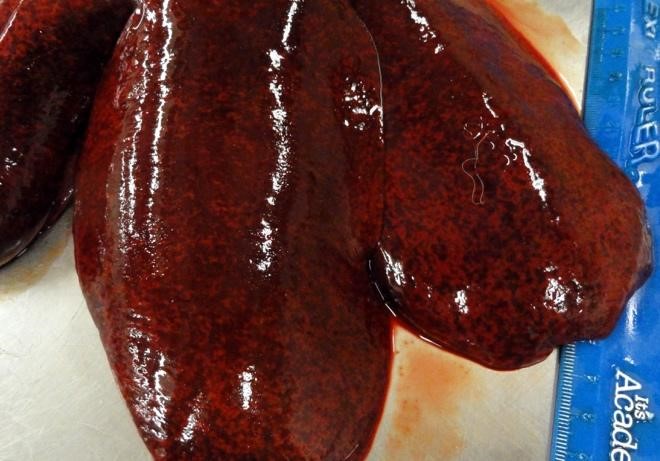
Additional pockets of subcutaneous hemorrhage were present over the rib cage and in the left jugular groove. Dry mucus was observed in the left conjunctiva. The spleen was enlarged and weighed 0.47% body weight (the animal was euthanized. The liver was 1.8% body weight (normal 3-4%), was mottled, extremely firm and had acute capsular margins. Most areas of tissue were firmer than expected, but others were soft, when pressure was applied across a tissue slice. The kidneys were congested and swollen.
Laboratory results: ALT values were >2000; ALP, GGT and total bilirubin were also elevated.
The animal was serologically positive for antibody against both Ehrlichia canis and Rickettsia rickettsia. PCR tests did not detect adenoviral, influenza of leptospiral antigens. Positive results were obtained for parvovirus and distemper. Toxicologic testing failed to detect arsenic or aflatoxins.
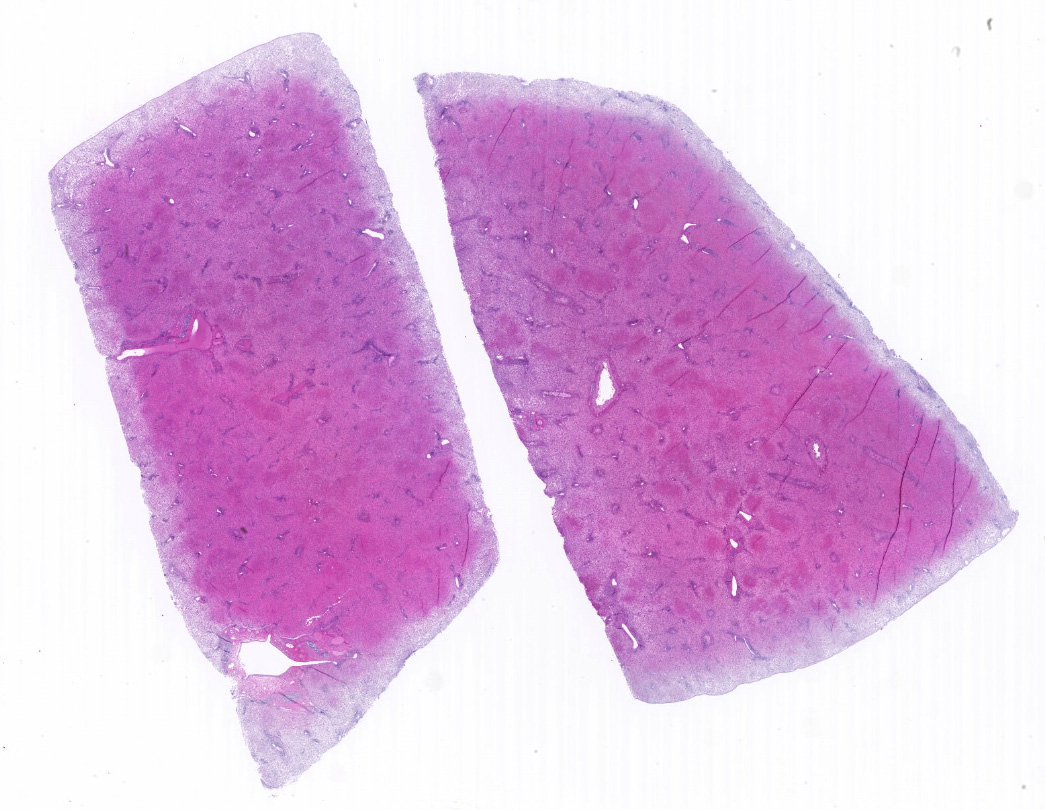
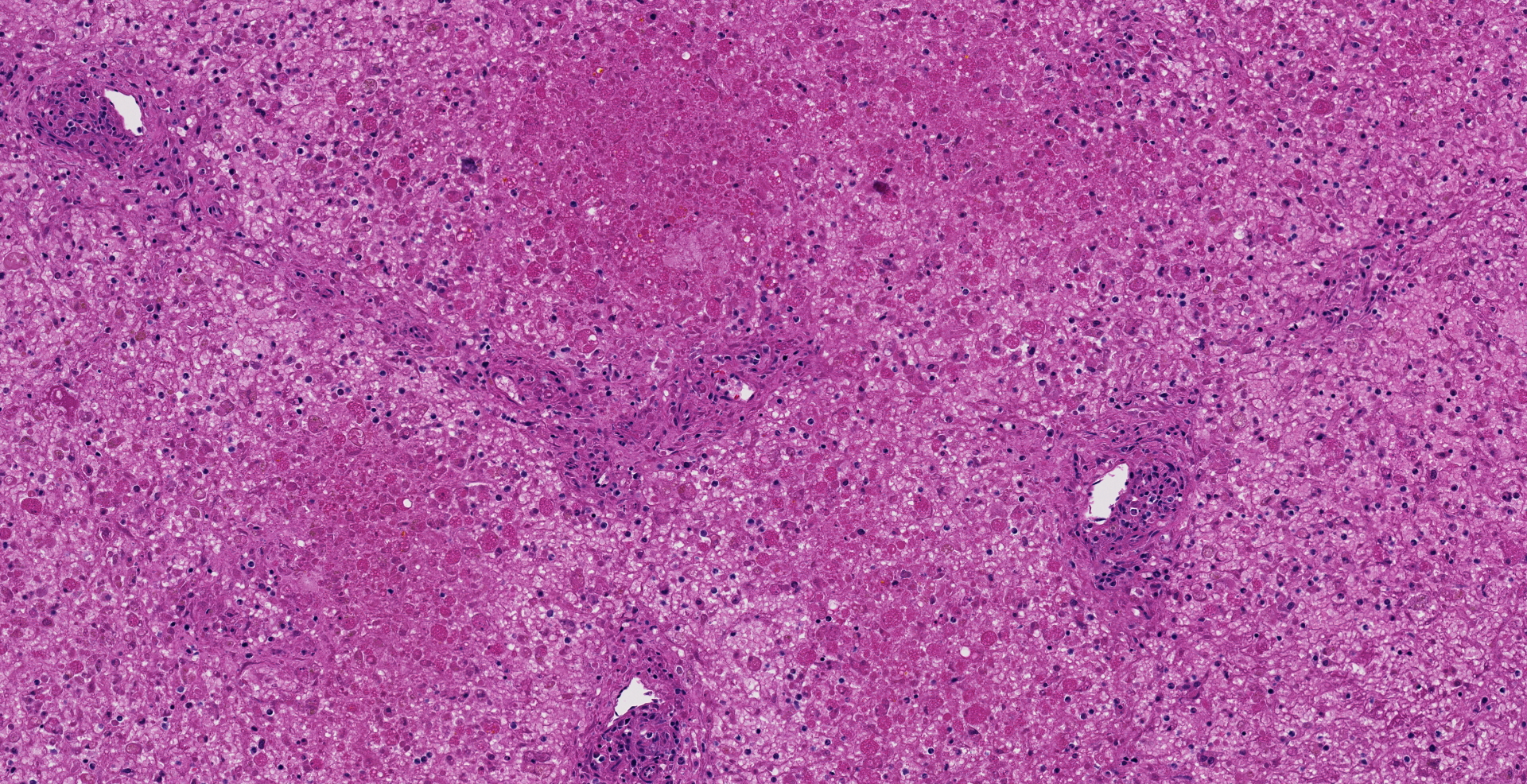
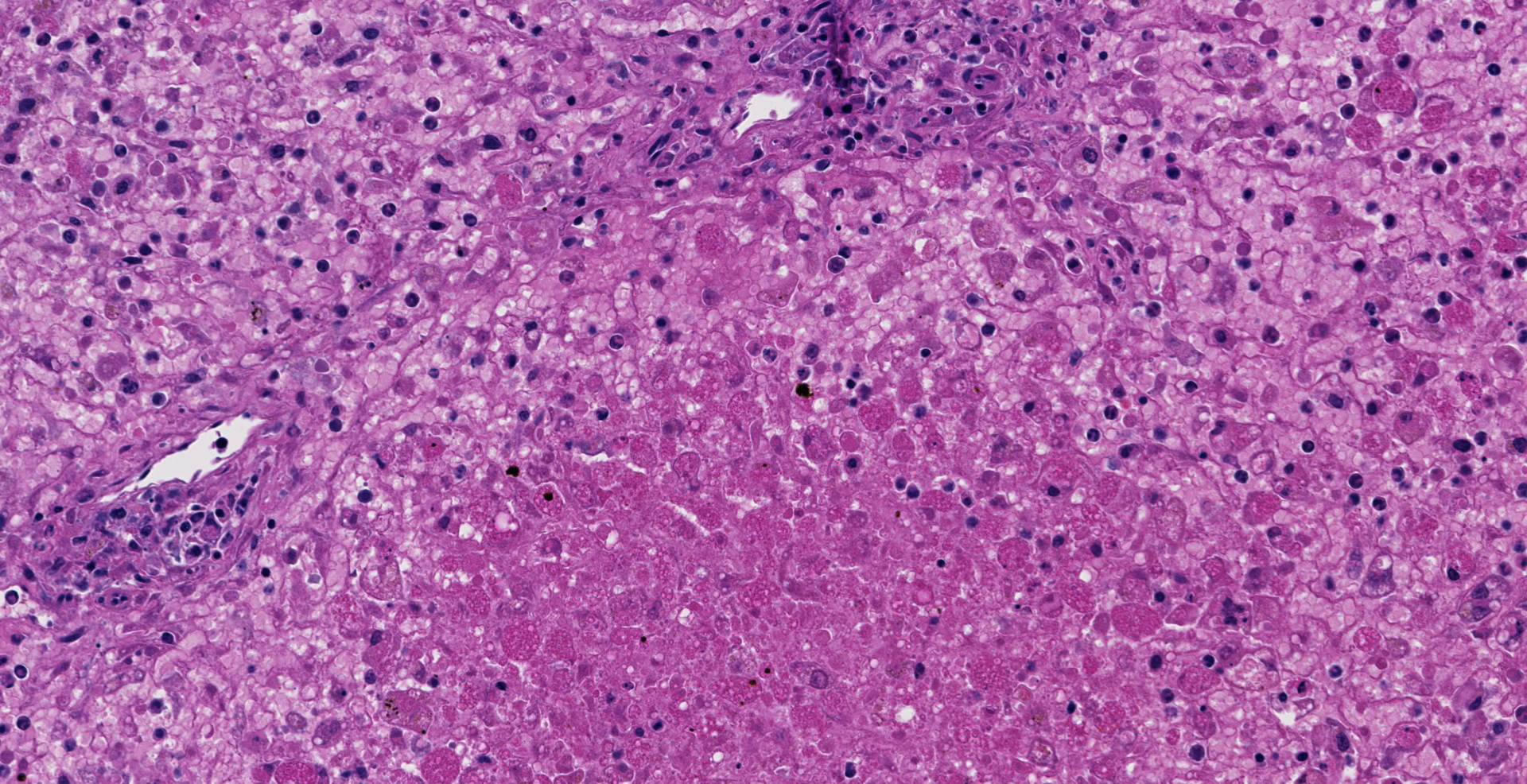
Microscopic description: The liver is collapsed and architecturally only bile ducts and a few lipid-rich, degenerating, rounded hepatocytes remain associated with the sinusoids. Necrotic cells frequently have pyknotic nuclei and vacuolated cytoplasm containing bile pigment is common amongst them. Scattered mixed leukocytes remain in the sinusoids or in the adventitia of portal areas. Lymphocytes and plasma cells are particularly numerous in portal triads. Collecting veins and sinusoids focally contain thrombi.
Contributor's morphologic diagnosis: Massive hepatic necrosis and loss, with periportal hepatitis
Contributor's comment:
Potentiated sulfonamides, combined with trimethoprim have become increasingly used to treat a variety of bacterial infections, especially those of the lung, skin and urinary tract in dogs. The two drugs are synergistic in inhibiting folate synthesis, inhibiting two steps of the pathway.
There are several manifestations of TMS toxicity in dogs, including anemia, non-septic arthritis, erythema multiforme-like skin syndrome and liver disease.14,16 Hepatopathy is the most frequently lethal manifestation of toxicity. Cholestasis and/or massive or sub-massive hepatic necrosis are commonly found.12,15 In some instances, lymphoplasmacytic infiltrates occur in lesions. Dogs can show multiple manifestations of toxicity. The drug dose typically is not excessive.
Illness commonly follows exposure by several days, so that the animal may not be currently taking the drug or be near the end of treatment. If surviving dogs are re-exposed to sulfa drug (with or without trimethoprim), recurrent disease is likely. It is thought that metabolic intermediates from drug detoxification produced by microsomal cytochrome P450 result in either a nitrosylated toxic metabolite or a hapten, perhaps one that binds to membranes and incites immune reaction that is lethal to the cell.
Onset of disease can occur near the end or after the antibiotic course, but disease extends beyond withdrawal of the drug. Some dogs that are affected have been given the drug previously. Clinicopathologic findings include increased alanine amino transferase, bilirubin, and alkaline phosphatase.11
Breed predispositions occur but vary between studies.4,5,14 At least one Saint Bernard has been reported with fatal liver disease, and Dobermans appear over-represented. The finding of related animals becoming ill in this case is striking.
The mechanism of toxicity is somewhat explored. Dogs do not have acetyl-methyl transferase activity, which is credited with producing the major non-toxic metabolite in people.13 The alternative pathway is biotransformation by hydroxylation and then reduction to produce a nitrosylated reactive product. The lack of N-acylation occurs in all dogs, but Doberman dogs, including an affected dog, had cells with intrinsic in vitro toxicity at lower dose indicative of a second mechanism.5
A variety of other drugs can also cause hepatic toxicity, including anesthetics halothane and methoxyflurane, anticonvulsants and others. Consumption of natural toxins such as microcytin can also result in similar lesions.
Contributing Institution:
Veterinary Medical Diagnostic Lab
University of Missouri
1600 East Rollins Street
Columbia MO 65211
JPC diagnosis:
Liver, hepatocytes: Necrosis, massive, diffuse, with marked hemorrhage.
JPC comment:
Research on adverse reactions in humans to sulfa drugs reported from 1968 to 1988 illustrated several key points. The research centered on using sulfa drugs as a primary or adjunct therapy in the treatment of malaria, as the effectiveness of chloroquine waned. Reported reactions from sulfa drug therapy included blood dyscrasias (15%), skin lesions (45%), and liver related disease (7%). Drugs in the co-trimoxazole family accounted for 67% of all reported adverse reactions, which includes the trimethoprim/sulfamethoxazole family of drugs, still widely used in veterinary medicine. This category of sulfa drug was also responsible for 60% of the case fatalities in this reported period. There is also an association between longer sulfa drug half-life and increased case fatality rate.1
This case also provides an opportunity to review causes of acute massive hepatic necrosis in domestic species. Other causes of massive hepatic necrosis include other drugs, toxins that damage the hepatocyte cytoskeleton, the inhibition of RNA synthesis, iron toxicity, diet, and viruses.
- Carprofen in dogs and diazepam in cats has been reported to cause idiosyncratic diffuse and massive hepatic necrosis at therapeutic doses. Diclofenac causes similar idiosyncratic liver injury to humans.6
- Microcystis aeruginosa, a freshwater cyanobacteria, creates hepatotoxin microcystin-LR, which inhibits hepatocellular protein phosphatases 1 and 2A, disrupting the cytoskeleton.3
- Amanita sp. mushrooms create the toxin phalloidin, which binds to actin and disrupts the cytoskeleton.3
- Marine dinoflagellates create pectenotoxins, which directly damage the hepatocyte cytoskeleton.3
- Aflatoxin (B1, B2, G1, and G2) cause widespread hemorrhage and massive hepatic necrosis, especially in young animals.6
-
Amanita sp. mushrooms
elaborate ![]() -,
-, ![]() -,
-, ![]() -, and
-, and ![]() -amanitin toxin,
which are potent inhibitors of RNA-polymerase, effectively halting RNA
synthesis in hepatocytes.6,8
-amanitin toxin,
which are potent inhibitors of RNA-polymerase, effectively halting RNA
synthesis in hepatocytes.6,8
- Both iron-dextran intoxication of piglets, and ferrous fumarate in foals have been described as causes of massive hepatic necrosis.2
- Hepatosis dietetica in pigs is incompletely understood but is likely related to deficiency of vitamin E and/or selenium.6
- Theiler's serum hepatitis in horses may be caused by a parvovirus, though research continues to build upon the body of knowledge.7
- Sulawesi tortoise adenovirus-1 (STAdv-1) was discovered to cause mortality in Sulawesi tortoises and has the capacity for interspecies transmission and infection.9
- Rift Valley Fever virus results in multifocal to massive hepatic necrosis in aborted lamb fetuses.10
Distinguishing between these causes can be difficult, and signalment and clinical history can provide the insight needed. This case highlights the need for additional information and analysis of lesions of other systems.
References:
1. Bjorkman A, Phillips-Howard PA. Adverse reactions to sulfa drugs: implications for malaria chemotherapy. Bulletin of the World Health Organization. 1991; 69(3) 297-304.
2. Brown DL, Van Wettere AJ, Cullen JM. Hepatobiliary System and Exocrine Pancreas. In: Zachary JF ed. Pathologic Basis of Veterinary Disease, 6th ed. St. Louis, MO: Elsevier. 2017: 451.
3. Cattley RC, Cullen JM. Liver and Gall Bladder. In: Wallig MA, Haschek WM, Rousseaux CG, Bolon B eds. Fundamentals of Toxicologic Pathology, 3rd Ed. San Diego, CA: Elsevier. 2018: 149.
4. Cribb AE. Idiosyncratic reactions to sulfonamides in dogs. J Am Vet Med Assoc. 1989;195(11):1612-1614.
5. Cribb AE, Spielberg SP. An in vitro investigation of predisposition to sulfonamide idiosyncratic toxicity in dogs.
6. Cullen JM, Stalker MJ. Liver and Biliary System. In: Maxie MG ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol. 2. St. Louis, MO: Elsevier. 2016: 258-351.
7. Divers TJ, Tennant BC, Kumar A, et al. New parvovirus associated with serum hepatitis in horses after inoculation of common biological product. Emerg Infect Dis. 2018; 24(2): 303-310.
8. Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc Natl Acad Sci U S A. 2007;104(48):19097-19101.
9. Rodriguez CE, Duque AMH, Steinberg J, Woodburn DB. Chelonia. In: Terio, KA, McAloose, D, St. Leger, J, eds. Pathology of Wildlife and Zoo Animals. San Diego, CA: Elsevier. 2018: 840-841.
10. Schlafer DH, Foster RA. Female Genital System. In: Maxie MG ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol. 3. St. Louis, MO: Elsevier. 2016: 439-440.
11. Thomson GW. Possible sulfamethoxazole/trimethoprim-induced hepatic necrosis in a dog. Can Vet J. 1990;31:530.
12. Thornberg LP. A study of canine hepatobiliary diseases, part 5: drug-induced hepatopathies. Comp Anim Pract. 1988;2(6):17-21.
13. Trepanier LA. Idiosyncratic toxicity associated with potentiated sulfonamides in the dog. J VET Pharmacol Therapy. 2004;27:129-138.
14. Trepanier LA, Dauhof R, Toll J, Watrous D. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfas. J vet Intern Med. 2003;17:647-652.
15. Twedt DC, Diehl KJ, Lappin MR, Getzy DM. Association of hepatic necrosis with trimethoprim sulfonamide administration in 4 dogs. J Vet Intern Med. 1997;11(1):20-23.
16. Werner LL, Bright JM. Drug-induced immune hypersensitivity disorders in two dogs treated with trimethoprim sulfadiazine: case reports and drug challenge studies. J Amer Anim Hosp Assoc 1983;19:783-790.