Signalment:
Four-month-old female
NOD.
Cg-PrkdcscidIl2rgtm1Wjl/SzJ mouse, (
Mus
musculus).Animal presented
moribund after receiving a xenotransplant.
Gross Description:
Discolored
nodules were visible on the kidney. Secondary lymphoid tissues were markedly
reduced in size consistent with the phenotype of the strain.
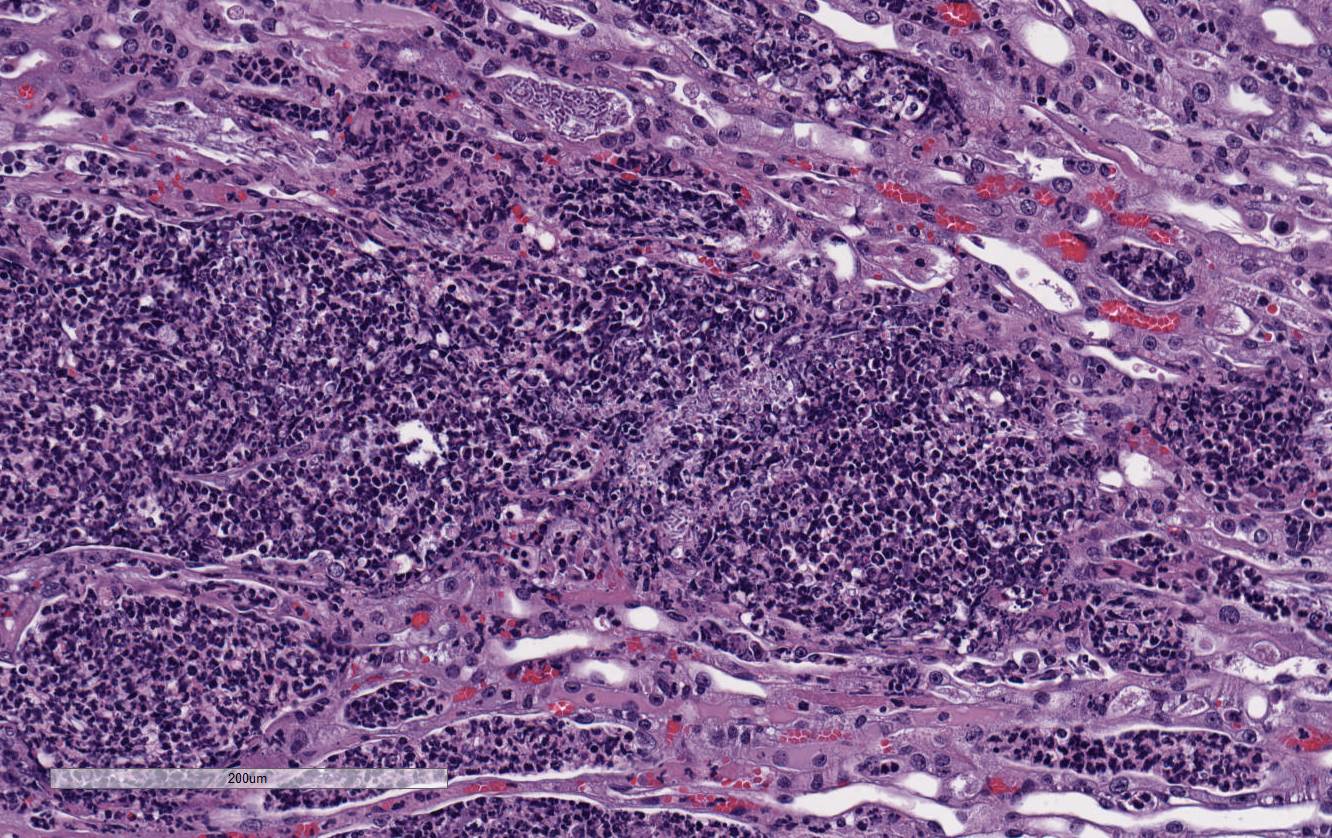
Histopathologic Description:
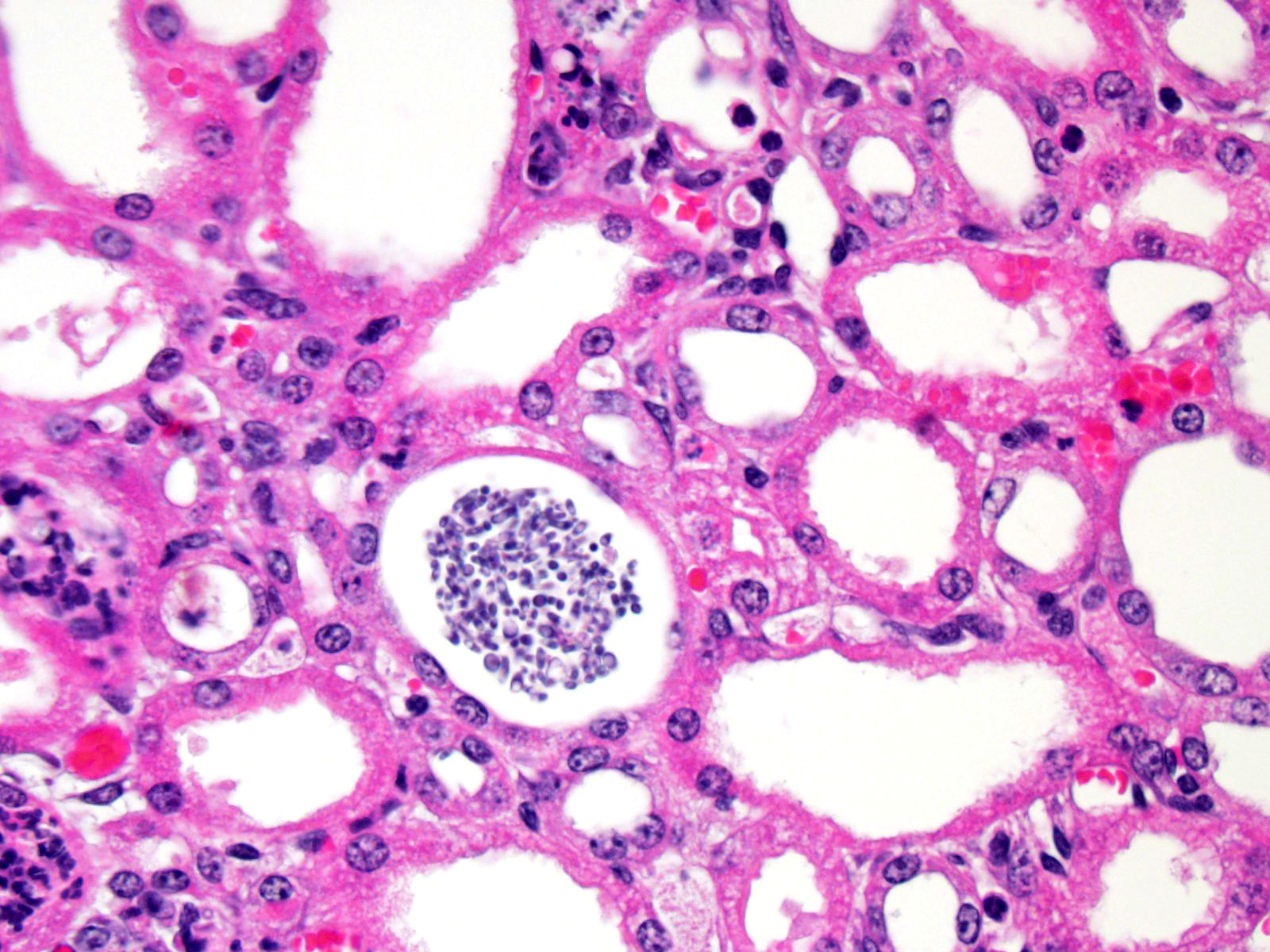
There
is marked, multifocal to coalescing neutro-philic inflammation and necrosis
arranged in an embolic pattern and which distorts the overall structure of the
kidneys as viewed subgrossly. Inflammation is centered on 0.5um wide X
8.0-10.0uM long fungal organisms that form non-branching hyphae and pseudohyphae.
Organisms and the accompanying inflammatory response involve predominately
vascular and perivascular, interstitial spaces and the tubules with limited
extension into the kidney parenchyma. Affected renal tubules are variably
ectatic and contain combinations of serocellular debris and acellular
eosinophilic material. The renal interstitium is variably expanded by
inflammation and reactive fibroblasts, especially where inflammation is
present. The adjacent sections of liver are unremarkable. Yeast forms are
also observed in the following locations: Both ears, heart, and the stomach.
Morphologic Diagnosis:
Kidney: Tubulointerstitial nephritis, suppurative,
bilateral, acute, severe with intratubular fungal organisms most consistent morphologically
with
Candida albicans.
Lab Results:
Microbiology,
Aerobic bacterial culture of both tympanic bullae:
Klebsiella pneumonia. Microbiology,
Aerobic bacterial culture of blood:
Staphylococcus xylosus.
Condition:
Systemic candidiasis
Contributor Comment:
Non-obese
diabetic (NOD)/severe combined immune deficiency (SCID)/IL2rγnull
(NSG) mice represent a severely immunocompromised mouse strain that is an
important tool for xenotransplantation studies using patient-derived tissues.
This strain is deficient in mature B cells, mature T cells, and natural killer
(NK) cells. Additionally, macrophages and dendritic cells are defective
regarding their functions.
5 While the severe immunodeficiency trait
of the strain is useful for avoiding immune-mediated host rejection of foreign
cells, this compromised status leaves NSG mice susceptible to a number of
opportunistic pathogens. Three commonly encountered opportunistic
pathogens of immuno-compromised laboratory mice are either observed in
tissue lesions or are isolated from body fluids of this NSG mouse including
Candida
albicans,
K. pneumoniae and
S. xylosus.
Candida albicans , the organism
observed in the renal lesions, is a common microbial component of the
gastrointestinal tracts of mice and other species that is held in check by
aspects of both the innate and adaptive immune responses.
3 The
ability of different laboratory mouse strains and man to contain any disease
that may be induced by
C. albicans is often related to having the
appropriate T-cell-directed phagocytic responses.
1 Interestingly the
depletion of T lymphocytes in the human and mouse generally results only in a
severe mucosal overgrowth of this yeast. Furthermore, neither nude nor
NOD-SCID mice have shown increased susceptibilities to developing systemic
candidiasis, which indicate that the innate immune system offers protection, as
well.
1 However, impaired NK cell function appears to be essential
for immune-mediated signaling that shields against developing systemic
candidiasis. Therefore, severely immuno-suppressed hosts, such as the NSG
mouse, are uniquely susceptible to systemic disease induced by
C. albicans
infection.
4
JPC Diagnosis:
1. Kidney:
Pyelonephritis, suppurative, acute, multifocal, marked with pseudohyphae and
yeast, NOD.
Cg-PrkdcscidIl2rgtm1Wjl/SzJ mouse,
Mus musculus.
2. Liver: No
significant lesions
Conference Comment:
In addition to being globally immuno-deficient and uniquely susceptible to a
variety of opportunistic infections, non-obese diabetic (NOD)/severe combined
immune deficiency (SCID; NSG) mice are selectively bred as an animal model for
type I diabetes mellitus (DM) in humans.
3 In NOD mice, DM develops as
a result of spontaneously developing autoimmune insulitis in the pancreatic
islets cells. The onset of DM is associated with a moderate glycosuria and a
non-fasting hyperglycemia, which may be important for the proposed pathogenesis
of this lesion discussed below.
3 As mentioned by the contributor, NOD-SCID
mice have functional defects in macrophages, dendritic cells, natural killer
(NK) cells, NKT cells, regulatory CD4+CD25+ cells, and are C5a deficient. The
susceptibility of these mice to develop DM is much higher in a sterile
environment.
3
Virulence of
C. albicans is dependent on adherence of the
organism to mucosal epithelial cells mediated by its virulence factor, adhesin.
The important components of adhesins are agglutinin-like sequence (ALS)
proteins and hypha-associated GPI-linked protein (Hwp1).
6,7
Superficial (localized) candidiasis produces relatively mild lesions in skin
and mucous membranes while systemic (disseminated) candidiasis may involve any
organ with the kidneys, heart valves, CNS and lungs most commonly affected. Immunosuppression,
cytotoxic chemotherapy, diabetes mellitus, long-term glucocorticoid therapy,
prolonged use of broad-spectrum antibiotics, or disruption of mucosal barriers predispose
to infection. In addition,
C. albicans can exist as yeast, pseudohyphae,
or true hyphae depending on environmental conditions and temperature. This polymorphism
enables
C. albicans to infect a variety of tissues throughout the body.
6,7
The yeast form has been implicated in disseminated systemic infection while
hyphae and pseudohyphae have stronger adherence and invasiveness due to the
expression of ALS adhesins, catalases, and protein hydrolases, such as secreted
aspartyl proteinases (SAP), which also supply the pathogen nutrients through
protein degradation.
6,7
Conference
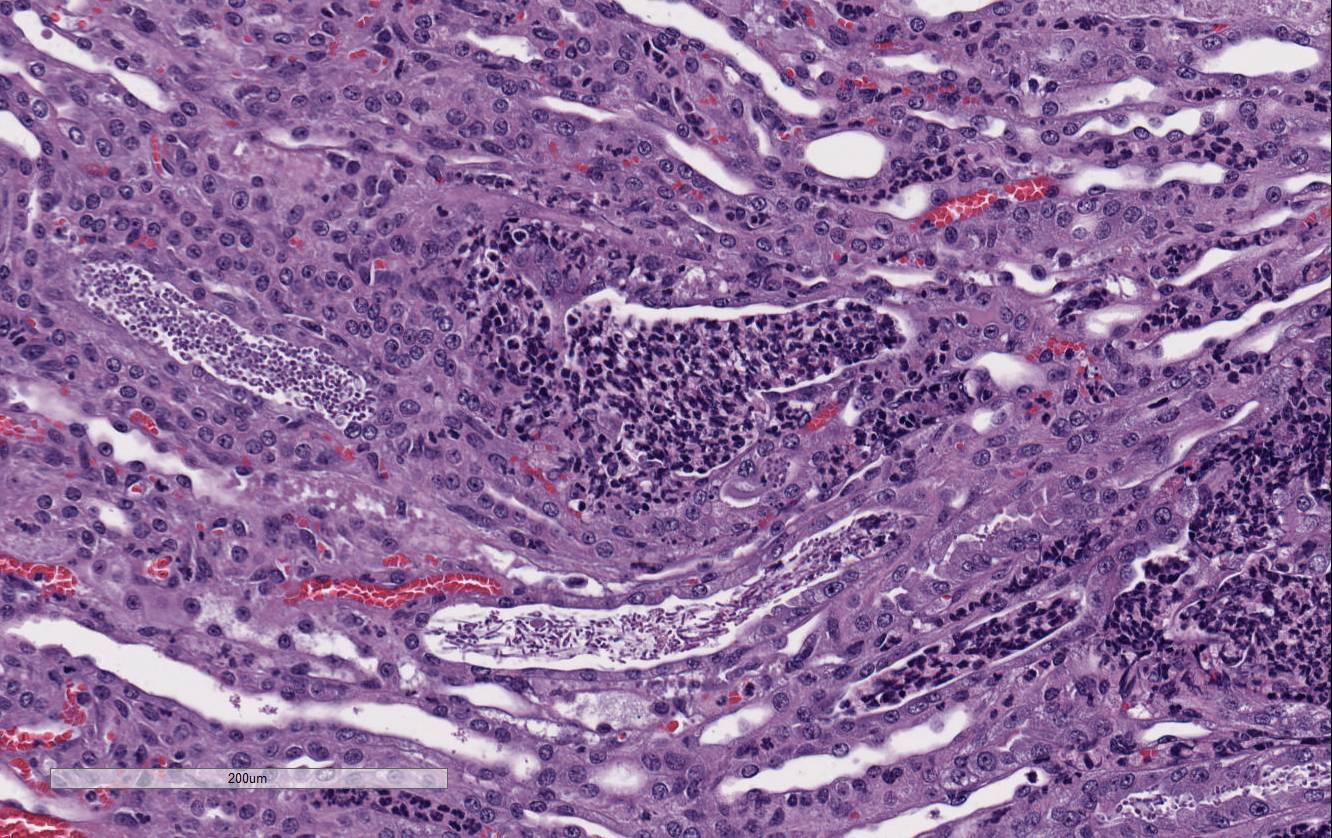
participants noted large numbers of
Candida sp. pseudohyphae and yeast
admixed with and surrounded by suppurative inflammation in the renal pelvis as
well as filling and expanding renal tubules in both the cortex and medulla.
Occasionally tubules rupture and the inflammation extends into the adjacent
renal interstitium. Participants agreed that this pattern of inflammation is
consistent with pyelonephritis and retrograde extension of exudate into the renal
tubules and renal parenchyma. The most common cause of pyelonephritis is via
ascending infection from the lower urinary tract.
2 Opportunistic
pathogens, such as
Candida albicans, can commonly ascend into the kidney
from the lower urinary tract, especially if there is glycosuria in a severely
immuno-compromised and diabeteic animal. Females are predisposed to ascending
urinary tract infections and pyelonephritis due to their relatively shortened
urethra compared to males.
2 Alternatively, hematogenous suppurative
and embolic nephritis usually results in multiple microabscesses within the
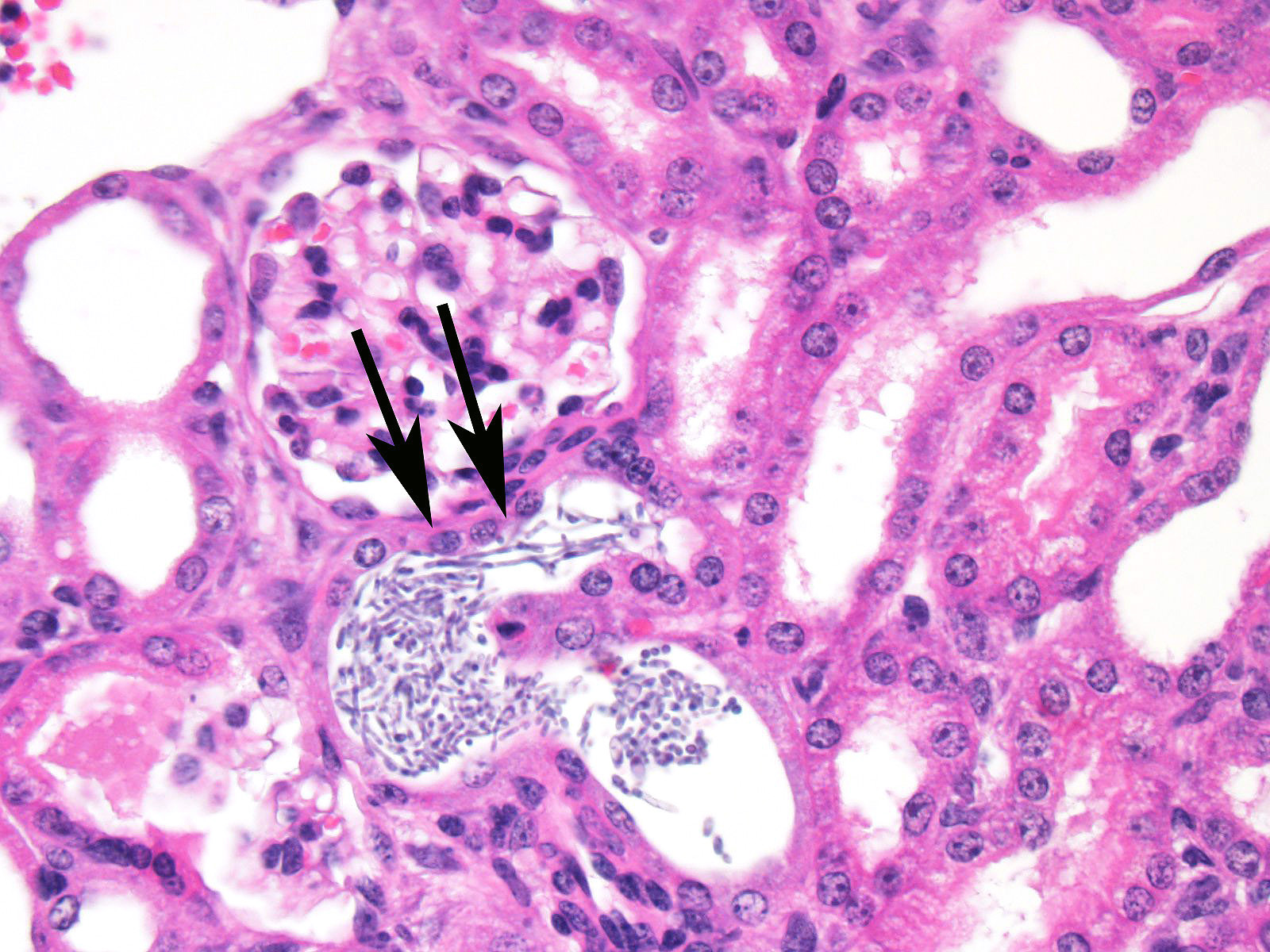
glomeruli, multifocal vascultitis, and hemorrhage within the medulla. In this
case, conference participants noted perivascular in-flammation, but not
significant vasculitis or glomerular lesions.
2
References:
1. Ashman,
R, Farah, C, Wanasaengsakul, S, Hu, Y, Pang, G, Clancy, R. Innate versus
adaptive immunity in
Candida albicans infection.
Immunol Cell Biol.
2004; 82(2):196-204.
2. Percy DH, Barthold SW.
Pathology of Laboratory Rodents and
Rabbits, 4
th ed. Ames, IA: Blackwell Publishing; 2016:4,79.
3. Quintin
J, Voigt J, Van der Voort R, et al. Differential role of NK cells against
Candida
albicans infection in immunocompetent or immunocompromised mice.
Eur J
Immunol. 2014; 44(8):2405-14.
4. "'>Shultz
L, Yoriko S, Najima Y, et al. Generation of functional human T-cell subsets
with HLA-restricted immune responses in HLA class I expressing
NOD/SCID/IL2rγ
null humanized mice.
Proc Natl Acad Sci
USA. 2010; 107:13022-13027.
5. Uzal
FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed.
Jubb,
Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed.
Philadelphia, PA:Elsevier; 2016:202.
6. Voon KC, Lee TY, Rusliza B, Chong PP. Dissecting
Candida
albicans infection from the perspective of
C. albicans virulence and
omics approaches on host-pathogen interaction: A review.
Int J Mol Sci.
2016; 17:1643.