Signalment:
Gross Description:
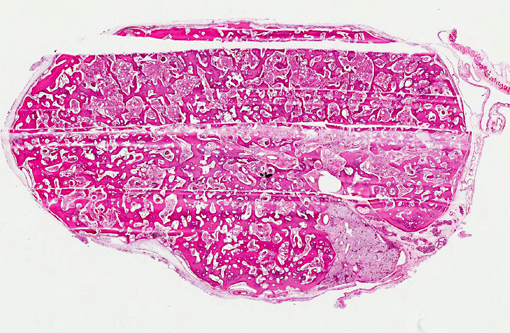
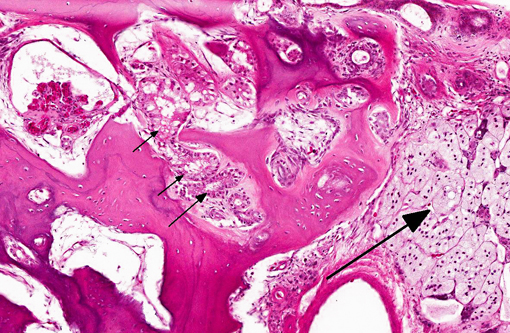
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Ectopic bone either arises from embryonic cell rests or by differentiation of adult, pluripotent mesenchymal cells into osteoblasts (osseous metaplasia).(2) Osseous metaplasia can occur anywhere uncommitted mesenchymal cells reside, including skeletal muscle, perivascular tissue, and connective tissue or sites of tissue regeneration and repair. It requires the influence of local osteogenic signals in an environment conducive to bone production.(3) Chronic ischemia, trauma, persistent hematoma, chronic inflammation, neoplasia, hypercalcemia, and hypervitaminosis D are among the factors known to stimulate osseous metaplasia.(4,5) While the pathophysiologic mechanisms leading to bone formation is not completely understood, paracrine signaling leading to the expression of bone morphogenetic proteins (BMPs) is likely a common key factor.(3) Most BMPs are members of the TGF beta superfamily and are critical signaling agents in normal development and differentiation and in the formation of new bone during fracture healing.(6) BMP expression has been demonstrated to play a role in neoplastic processes associated with ectopic bone formation, as well as experimental models of chronic inflammation.(7) In addition to non-committed mesenchymal cells, vascular endothelial cells(8) and pericytes(9) have also been implicated as potential cells of origin for osseous metaplasia. Interestingly, inactivating germline mutations of the α-subunit of the stimulatory G protein gene leads to subcutaneous and sometimes deeper ectopic bone formation in humans (Albright hereditary osteodystrophy) and in mice.(10) Osseous metaplasia is also seen in association with dystrophic cardiac and pulmonary mineralization in particular strains of mice where early events involve abnormal cellular calcium, mitochondrial alterations, and myocyte injury in the absence of elevation of serum calcium.(12,13)
While pathologic mineralization within the kidney is a not an uncommon finding in cases of chronic renal disease, the presence of abundant, trabecular bone in the renal parenchyma of this turtle is remarkable. Reports of spontaneous osseous metaplasia in the kidney are rare in the human medical literature and even rarer in the veterinary medical literature. In humans, ossified tissue in the kidney is associated with chronic interstitial nephritis, chronic ischemia, pyelonephritis, and papillary necrosis, and it is an uncommon nidus for renal calculus formation.(5,11) Contributing factors for renal osseous metaplasia observed in this turtle likely included chronic inflammatory stimulation and possibly calcium/phosphorous imbalance secondary to chronic renal dysfunction.Â
JPC Diagnosis:
1. Kidney: Osseous metaplasia, diffuse, severe, with renal tubular degeneration and necrosis.
2. Kidney: Nephritis, interstitial, lymphoplasmacytic, diffuse, moderate.
Conference Comment:
References:
1. Thompson K. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 1. 5th ed. Philadelphia, PA: Saunders Elsevier, 2007:1-184.
2. Myers R, McGavin M, Zachary J. Cellular adaptations, injury, and death: morphologic, biochemical, and genetic basis. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:2-59.
3. McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609619.
4. Landim FM, Tavares JM, de Melo Braga DN, da Silva JE, Bastos Filho JBB, Feitosa RGF. Vaginal osseous metaplasia. Arch Gynecol Obstet. 2009;279:381384.
5. Bataille S, Daniel L, Legris T, Vacher-Coponat H, Purgus R, Berland Y, Moal V. Osseous metaplasia in a kidney allograft. Nephrol Dial Transplant. 2010;25:37963798.
6. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;346:2637.
7. Rifas L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J Cell Biochem. 2006;98:706714.
8. Medici D, Olsen BR. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res. 2012;27:16191622.
9. Dayoub S, Devlin H, Sloan P. Evidence for the formation of metaplastic bone from pericytes in calcifying fibroblastic granuloma. J Oral Path Med. 2003;32:232236.
10. Huso DL, Edie S, Levine MA, Schindinger W, Wang Y, Harald J, Germain-Lee EL. Heterotopic Ossifications in a mouse model of Albright hereditary osteodystrophy. Plos One. 2011:6:e21755.
11. Fernandez-Conde M, Serrano S, Alcover J, Aaron JE. Bone metaplasia of urothelial mucosa: an unusual biological phenomenon causing kidney stones. Bone. 1996;18:289291.Â
12. Percy DH, Barthold SW. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Iowa State Press; 2007:94-95, 219.
13. Ernst H, Dungworth DL, Kamino K, Rittinghausen S, Mohr U. Nonneoplastic lesions in the lungs. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, eds. Pathobiology of the Aging Mouse. Washington, DC: ILSI Press; 1996:298.