CASE 3: 19080625 (4152936-00)
Signalment:
A 7-year-old, intact female, leopard tortoise (Stigmochelys pardalis)
History:
This animal had a one-month history of respiratory distress and nasal discharge.
Gross Pathology:
The oral cavity contains multiple off-white to light tan, confluent plaques on the lingual and gingival mucosa. Numerous, viable, beige, cylindrical, approximately 0.3 to 0.4 cm diameter ectoparasites (fly larvae) infiltrate the esophageal and peri-esophageal soft tissues at the entry of the esophageal tube. A red, 0.5 cm diameter, skin discoloration is present under the chin. The caudal coastal and vertebral carapaces are raised. Multiple cystic spaces, measuring up to 1-1.5 cm diameter, are observed in the left lung. These cystic structures are filled with a moderate amount of yellow-tan, clear and mucoid fluid. Numerous, up to 1 mm diameter, pale tan to white foci are evident on the surfaces of the spleen and liver. On cut section, these foci are scattered throughout the splenic and hepatic parenchyma. The stomach contains approximately 100 mLs of grey to brown, mucoid fluid. Two pale, raised, pinpoint foci are present on the mucosal surface of the stomach. The intestinal lumen contains a mixture of tan to pink, semisolid ingesta. Formed, dark green feces are present in the distal colon.
Laboratory results:
Aerobic culture of the lung yielded large numbers of Proteus spp., Enterococcus faecalis, Alcaligenes spp., Citrobacter braakii, and moderate numbers of Pseudomonas aeruginosa, Escherichia coli, Providencia spp., Enterococcus casseliflavus, and Morganella morganii
Microscopic description:
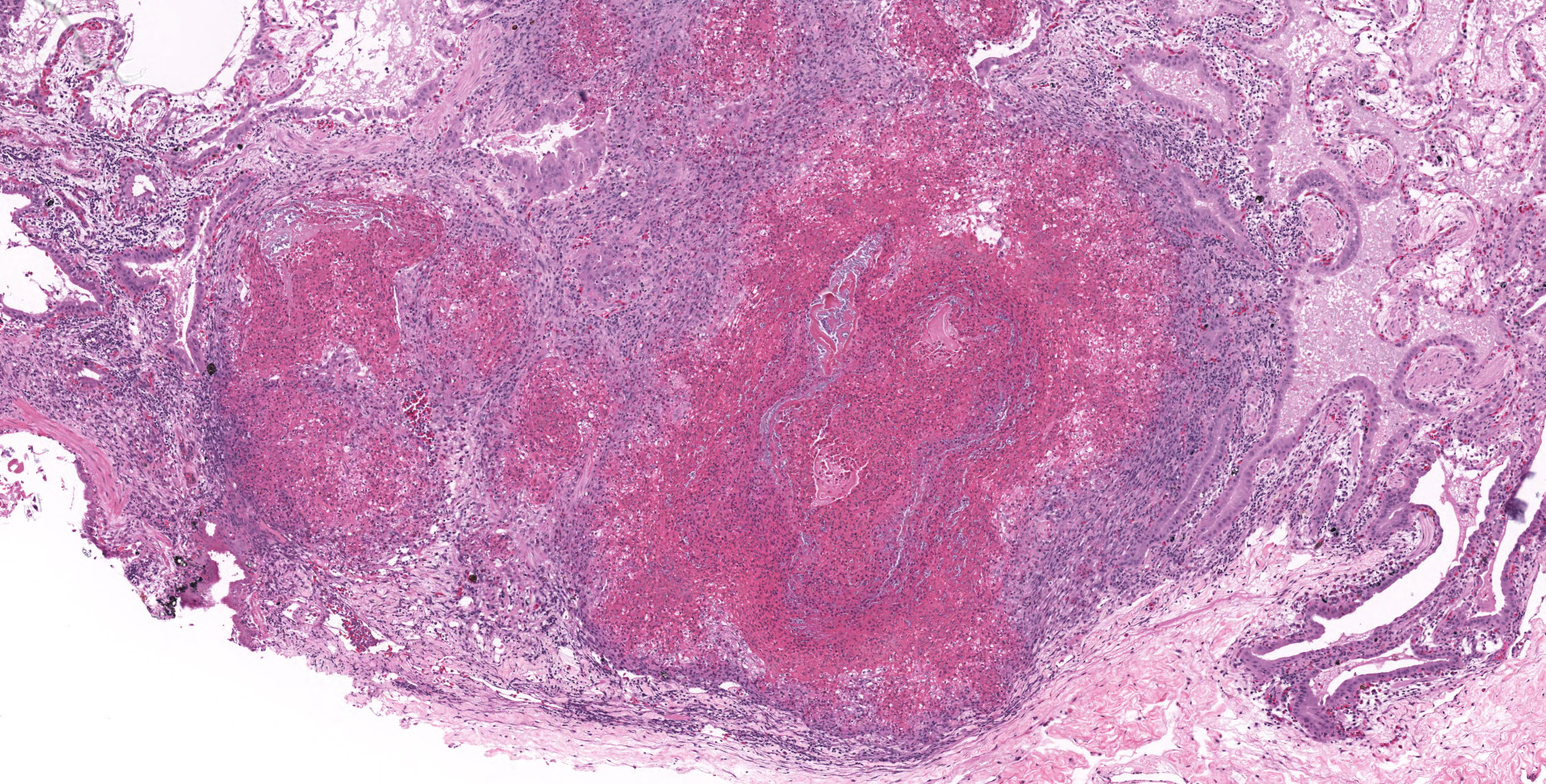
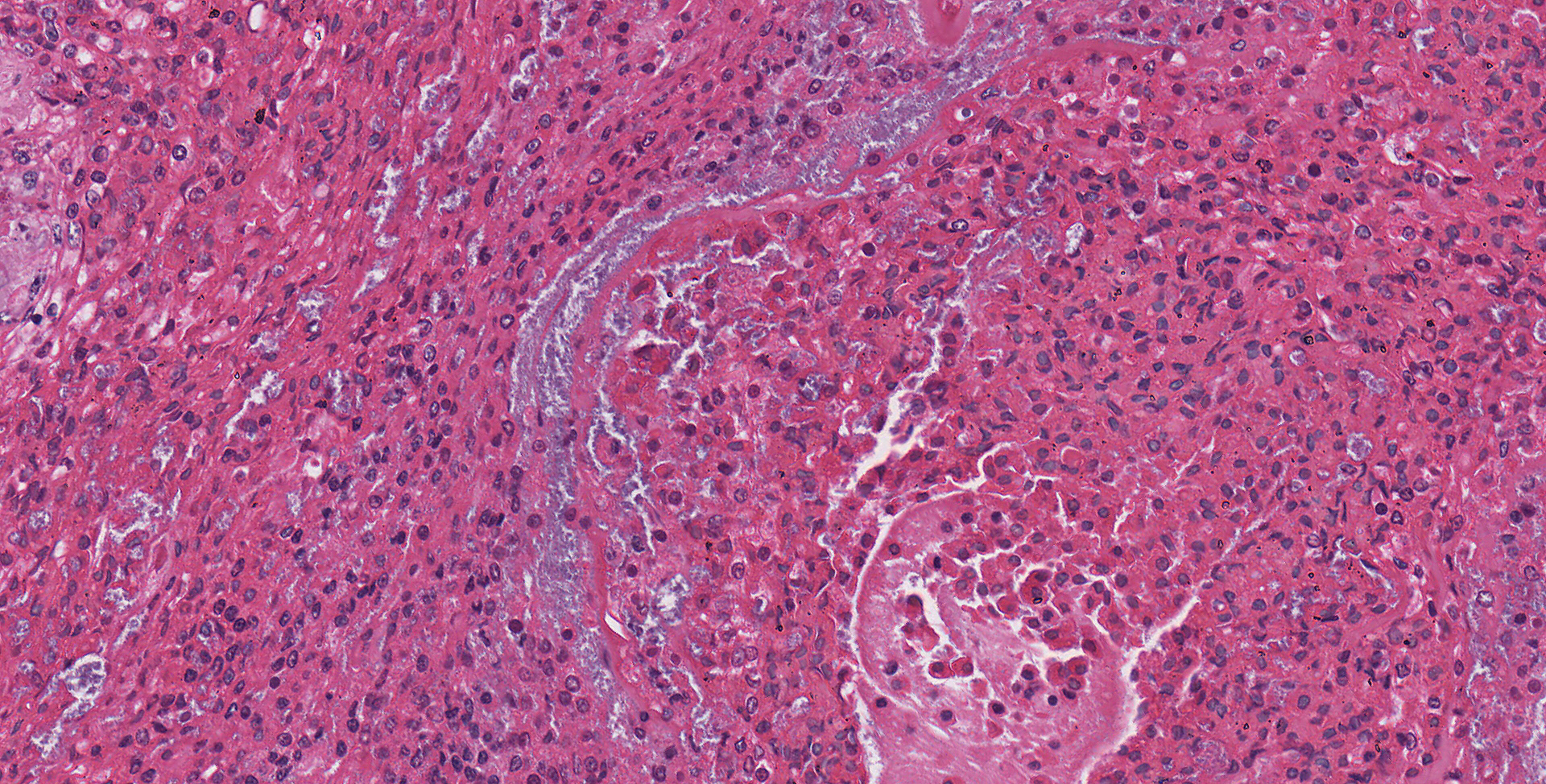
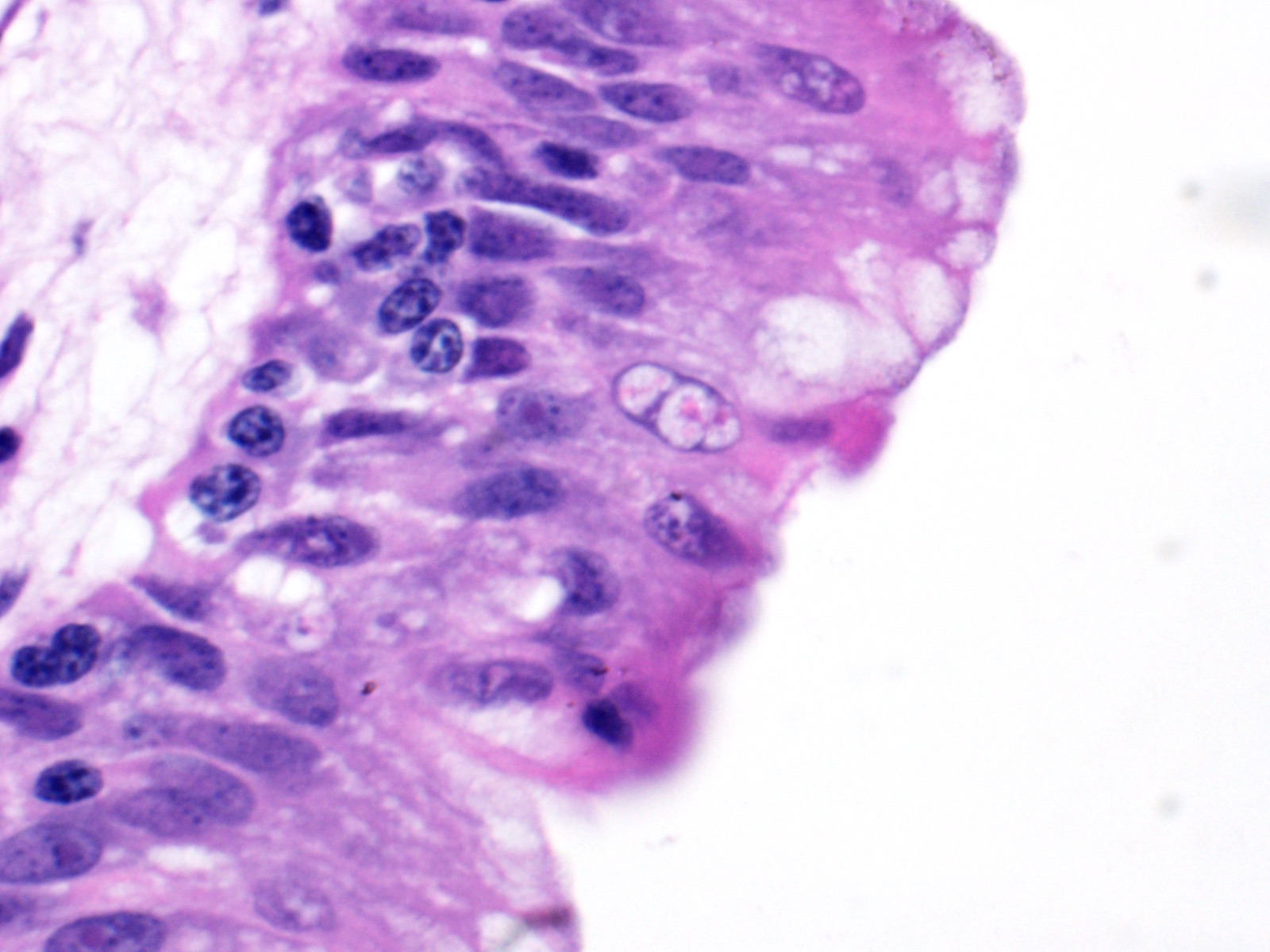
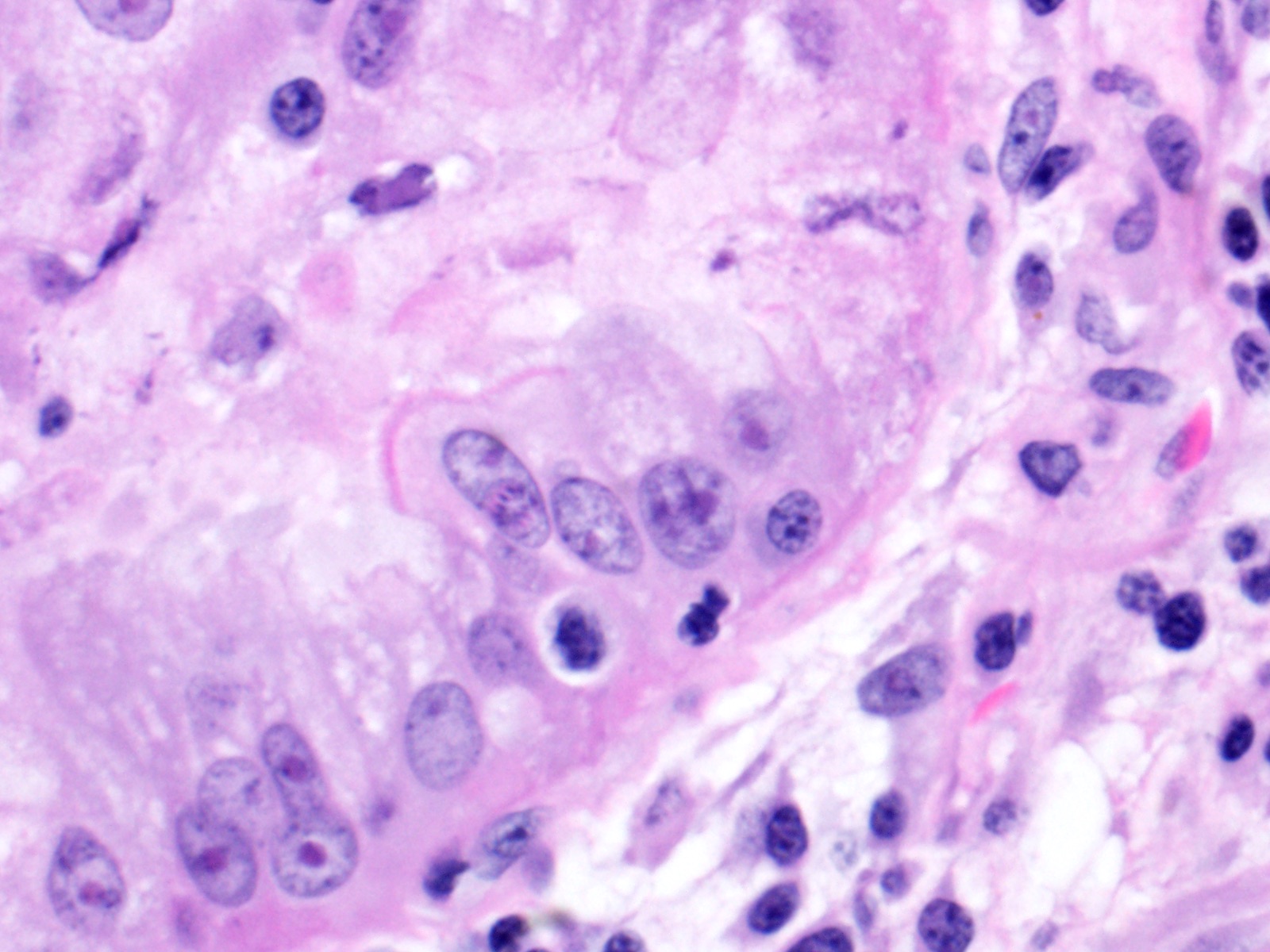
Lung: Moderately to markedly filling random bronchi and bronchioles, also with multifocal expansion of the interstitium, are multifocal to coalescing areas of necrosis, composed of massive amounts of cellular and nuclear debris, numerous degenerate heterophils, and myriad numbers of intralesional coccobacilli. Necrotic foci are sometimes surrounded by increased numbers of intact heterophils and fewer macrophages. Affected bronchi and bronchioles are lined by either intact and necrotic, or absent epithelium. In other less affected areas, the bronchial and bronchiolar epithelium remains intact, and is moderately to markedly hyperplastic, with occasional intranuclear protozoa at various stages of development (coccidia). Developmental stages present include: intranuclear ovoid to spherical gametes that are up to 5-6 µm in diameter with granular basophilic cytoplasm and eccentric nucleus; intranuclear spherical meronts that are 5-7 µm with numerous banana-shaped merozoites; and intraluminal unsporulated oocysts that are approximately 8-12 µm in diameter. Multifocally, ectatic respiratory lumina often contain slightly granular, proteinaceous cellular debris interspersed with coccidia at various stages of development.
Contributor's morphologic diagnosis:
Lung: Bronchointerstitial pneumonia, necrotizing, heterophilic and histiocytic, subacute, multifocal to coalescing, marked, with bronchi and bronchiolar epithelial hyperplasia with intranuclear protozoal coccidia, and intralesional coccobacilli
Contributor's comment:
The microscopic features of the lung are consistent with the entity described as Intranuclear coccidiosis of testudines (TINC), which is presumed to be the primary change in this case. A variety of bacteria were isolated from the lung but are likely resultant from opportunistic infection or environmental contaminants.
Infection of Testudines with intranuclear coccidia was first identified in the 1990s, and has now emerged as one of the most important infectious diseases affecting a variety of chelonian species.4,7 Reported species to date include radiated tortoise (Geochelone radiata), impressed tortoise (Manouria impressa), leopard tortoise (Geochelone pardalis), Travancore tortoise (Indotestudo forstenii), Bowsprit tortoise (Chersine angulate), Sulawesi tortoise (Indotestudo forsteni), Eastern box turtle (Terrapene carolina), Arakan forest turtles (Heosemys depressa), Spider tortoise (Pyxis arachnoides), flat-tailed tortoises (Pyxis planicauda), Galapagos tortoises (Chelonoidis nigra becki), marginated tortoise (Testudo marginata), Hermann's tortoise (Testudo hermanni), Greek tortoise (Testudo graeca), Russian tortoise (Testudo horsfieldi), Indian star tortoise, African spurred tortoise (Geochelone sulcate), and yellow footed tortoise (Chelonoidis denticulatus).2,3,5-8 The causative agent of TINC is a novel coccidia that has not been assigned to a genus as of yet; however, the 18S ribosomal RNA (rRNA) nucleotide sequencing analysis reveals that it is most closely related to Eimeria arnyi and Eimeriid coccidum, based on two separate studies.5,6
The route of transmission of TINC remains elusive, but direct, fecal-oral transmission is most likely, based on the finding of oocysts in the feces of TINC-positive tortoises, and successful infection of other tortoises following experimental oral inoculation.5 The unsporulated oocyst of this parasite is spherical, and measures 6-12 µm in diameter with a centrally located sporont.5,7 Sporulation of the oocysts requires 3-4 days, which is a crucial stage during the life cycle of TINC.5 The sporulated oocyst contains four sporocysts, which are thin and smooth walled, surrounding two sporozoites.5 Within the infected mammalian cell nuclei, one or more coccidia at variable stages of development can be detected. Trophozoites measure 2-5 µm in diameter.3,6 Meronts are up to 7 µm in diameter with numerous merozoites.3,6 Merozoites are banana shaped, and measure 4 X 1.5 µm with a single central to slightly3-7 eccentric nucleus.3,6 Macrogametes and microgametes are spherical and up to 6 µm in diameter.3,6
Clinical signs can be variable and include: anorexia, lethargy, weight loss, weakness, increased respiratory effort, mild chronic conjunctival erythema, bilateral ocular and nasal mucoid discharges, oronasal fistula, ascites, retained urine in the urinary bladder, severe cutaneous edema and/or ulceration.3,4,6,8 Death can occur within a few days after initial onset of clinical signs of disease.3,4 Useful antemortem diagnostic methods include cytologic examination of the nasal discharge, histologic examination of the affected tissue via biopsy, and molecular testing such as polymerase chain reaction (PCR) performed on swab specimens from the oral, ocular, nasal, and cloacal mucosa.4,6 Cytologic preparations generally include a predominance of lymphocytes and plasma cells, with fewer macrophages and granulocytes. Intranuclear coccidia can sometimes be detected within affected epithelia stained with Fite acid-fast, Periodic Acid-Schiff (PAS), or Wright-Giemsa.6
TINC is a systemic disease, and gross lesions can be observed within a diversity of organ systems to include: alimentary, respiratory, cardiovascular, urogenital, and integumentary.3-7 The common gross findings include pallor of mucous membranes, thick oral mucus, pseudomembranous enteritis, cloaca erythema, rhinitis, pulmonary congestion, pericardial and intestinal petechiae, firm kidneys with a gray to red mottled discoloration, splenic congestion, discolored liver, distended urinary bladder, and generalized subcutaneous edema.3-7 Histologically, lymphoplasmacytic inflammation and necrosis with intranuclear, or less frequently, intracytoplasmic and extracellular coccidial organisms are evident within the affected tissue.3,4,6,7 Various stages of coccidia are more commonly seen in the nucleus of respiratory epithelium, hepatocytes, biliary tree epithelium, pancreatic ductular and acinar cells, enterocytes, renal tubular epithelium, and urinary bladder epithelium, but they can also be detected in thyroid and adrenal glands, gonads, lymphoid organs, and skin.3,4,6,7 Quantitative PCR (qPCR) is a rapid and sensitive diagnostic option for confirmation of TINC in suspected cases.2
Contributing Institution:
Department of Veterinary Pathobiology
College of Veterinary Medicine
Oklahoma State University
https://vetmed.okstate.edu/veterinary-pathobiology/index.html
JPC diagnosis:
1. Lung: Pneumonia, interstitial, granulocytic, histiocytic, with bacterial colonies, type II pneumocyte hyperplasia, and interstitial fibrosis, leopard tortoise, chelonid.
2. Lung, bronchiolar and faveolar epithelium: Hyperplasia and lymphocytic inflammation, with intranuclear gamonts, intranuclear meronts, intracytoplasmic zoites, and extracellular oocysts.
JPC comment:
The concurrent bacterial pneumonia in this case likely contributed significantly to clinical signs. The inflammation is primarily centered on faveoli, indicating a likely hematogenous route of entry to the lungs. If the described lesions in the spleen and liver are similar in character to those in the lung, this animal was likely septic. However, without sections of other tissues, a definitive diagnosis can only be speculative.
The contributor provides an excellent summary of this emerging disease of testudines. Since submission, this unnamed coccidian has also been documented in the red-footed tortoise (Chelonoidis carbonaria). One interesting aspect of this disease is that all discovered cases have occurred in managed-care settings, to include recently imported animals. With no documented cases in wild chelonians, there may be either too few free-living chelonian cases investigated1,11, and/or there is a variable related to captivity that modulates disease in these animals.
Current recommended therapy includes antiparasitic medications, such as toltrazuril or ponazuril, though care should be taken not to conclude treatment prematurely. Chelonians that die following treatment have had fibrosis in areas previously infected by the apicomplexan, illustrating its potential long-term tissue damage to organs.1
Tortoises often enjoy a long life but remain susceptible to environmental changes. Lonesome George was the endling Pinta Island tortoise (Chelonoidis abingdonii), and lived on Pinta Island, a small island of the Galapagos Islands chain, of Ecuador. Discovered in in 1971, he remained the last tortoise in the face of environmental degradation from introduced feral goats. He was relocated to the Charles Darwin Research Station on a nearby island for his safety, but never successfully reproduced with females of closely related species. He died in 2012, an estimated 100 years old, still thought to be relatively young for a tortoise of his species.10
Giant tortoises are among the longest-lived vertebrates and are studied as models of longevity and age-related disease. A global genetic analysis was performed on Lonesome George following his death. For comparison, a genome of Aldabrachelys gigantea was also sequenced, as a related tortoise that diverged from Chelonoidis abingdonii approximately 40 million years ago. Across a number of genes, there were similar numbers of copies of tumor suppressor genes (PTPN11, P2RY8, PML, NF2, SMAD4, PRDM1) in these two tortoise species, and there were more copies than in the human genome. Similarly, across several genes associated with ageing (NEIL1, RMI2, EEF1A1, GAPDH), the tortoises' genomes had more copies of genes than the human genome, most of which contribute to DNA stability and result in fewer mutations over time. Continued research with these genomes may reveal novel therapeutics or interventions to increase human survival with increased health.9
References:
1. Adamovicz L, Allender MC, Gibbons PM. Emerging Infectious Dsiease of Chelonians: An Update. Vet Clin Exot Anim. 2020;23:263-283.
2. Alvarez WA, Gibbons PM, Rivera S, Archer LL, Childress AL, Wellehan JFX. Development of a quantitative PCR for rapid and sensitive diagnosis of an intranuclear coccidian parasite in Testudines (TINC), and detection in the critically endangered Arakan forest turtle (Heosemys depressa). Vet Parasitol. 2013;193:66?70.
3. Garner MM, Gardiner CH, Wellehan JFX, et al. Intranuclear coccidiosis in tortoises: Nine cases. Vet Pathol. 2006;43:311?320.
4. Gibbons PM, Steffes ZJ. Emerging Infectious Diseases of Chelonians. Vet Clin North Am - Exot Anim Pract. 2013;16:303?317.
5. Hofmannová L, Kvi?erová J, Bízková K, Modrý D. Intranuclear coccidiosis in tortoises ? discovery of its causative agent and transmission. Eur J Protistol. 2019;67:71?76.
6. Innis CJ, Garner MM, Johnson AJ, et al. Antemortem diagnosis and characterization of nasal intranuclear coccidiosis in Sulawesi tortoises (Indotestudo forsteni). J Vet Diagnostic Investig. 2007;19:660?667.
7. Jacobson ER, Schumacher J, Telford SR, Greiner EC, Buergelt CD, Gardiner CH. Case report of systemic coccidiosis in a radiated tortoise (Geochelone radiata). J Zoo Wildl Med. 1994;25:95?102.
8. Kolesnik E, Dietz J, Heckers KO, Marschang RE. Detection of Intranuclear Coccidiosis in Tortoises in Europe and China. J Zoo Wildl Med. 2017;48:328?334.
9. Quesada V, Freitas-Rodriguez S, Miller J, et al. Giant tortoise genomes provide insights into longevity and age-related disease. Nature Ecology and Evolution. 2019;3:87-95.
10. Raferty, Isolde. "Lonesome George, last-of-its-kind Galapagos tortoise, dies". MSNBC. Archived from the original on 2012-06-27. Retrieved 2020-08-23.
11. Rodriguez CE, Duque AMH, Steinberg J, Woodburn DB. Chelonia. In: eds Terio KA, McAloose D, St. Leger, J. Pathology of Wildlife and Zoo Animals. San Diego, CA:Elsevier. 2018:847:848.