Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 8
October 24, 2018
CASE I: Case 1 6981 R23 (JPC 41078788-00).
Signalment: Adult, female, N/A, Sciurus vulgaris, red Eurasian squirrel
History: A frozen, adult red squirrel was presented for postmortem examination as part of a surveillance scheme aiming to understand the causes behind the decline in red squirrel populations in the UK.4
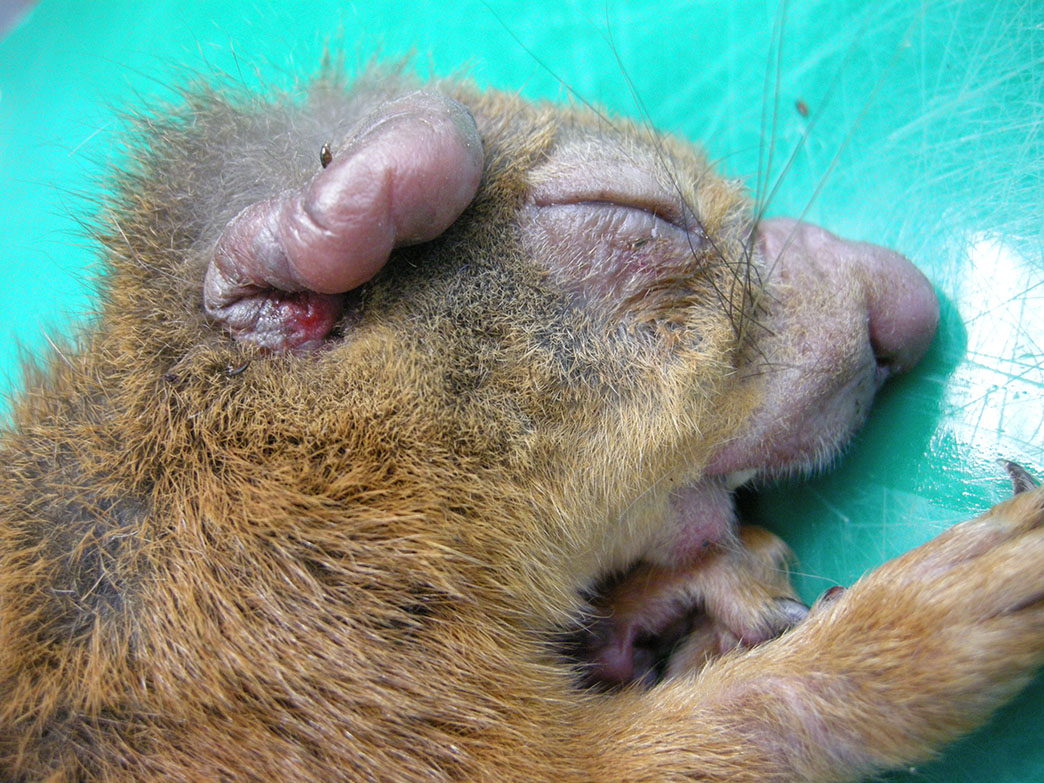
Gross Pathology: This squirrel had bilateral areas of alopecia and cutaneous swelling at the snout, lips, eyelids, pinna and the ventral and distal aspect of all limbs (figure 1). Internally, the thoracic cavity was filled by a moderate amount of pale pink, opaque fluid (chylothorax).
Laboratory results: Hsp65 PCR followed by sequencing6, and ulterior whole genome sequence work1 were positive for Mycobacterium lepromatosis.
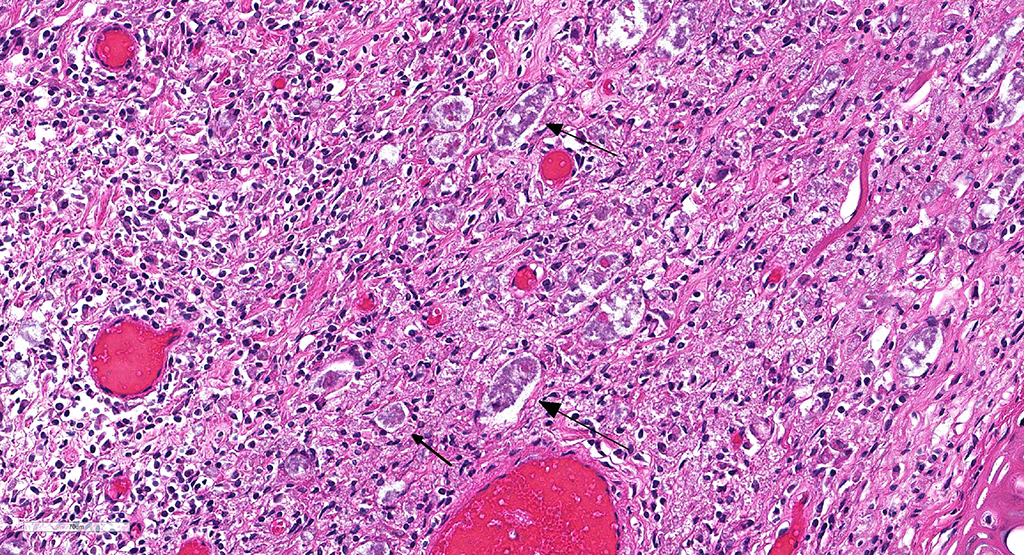
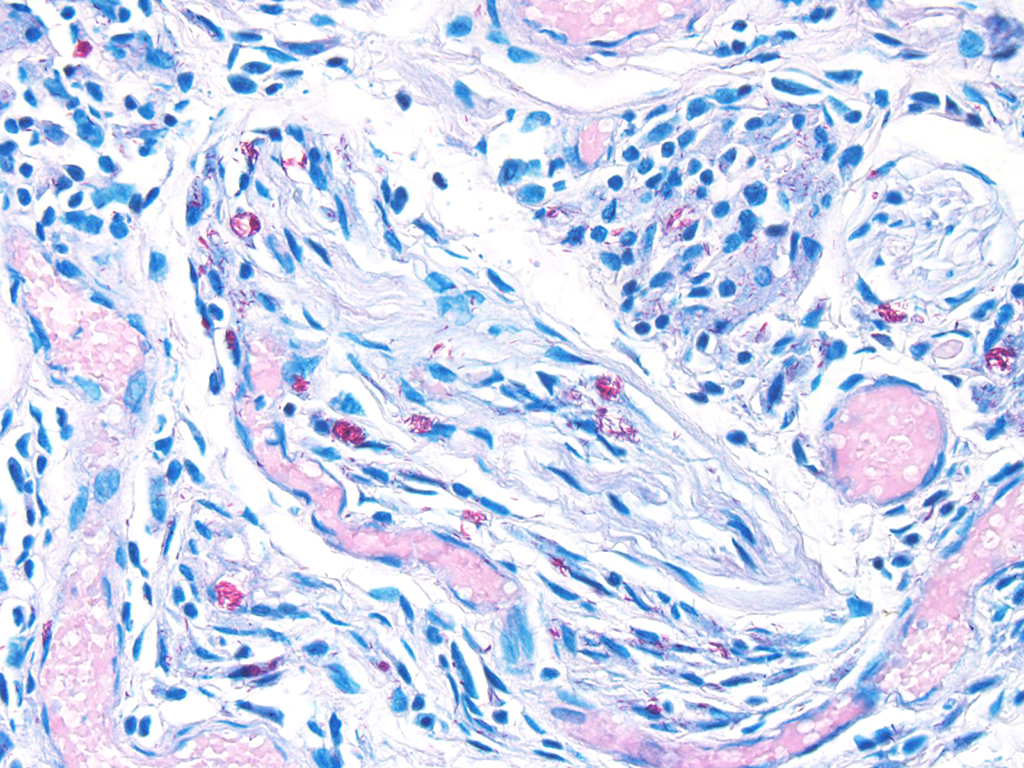
Microscopic Description: Description of the pinna only: At the tip of the pinna, extending from the aural cartilage to the superficial dermis, there is severe, focally extensive expansion by intercellular spacing (oedema) and severe infiltration by inflammatory cells. The latter are mostly foamy macrophages, with fewer lymphocytes and plasma cells and rare multinucleated giant cells. Scattered throughout this area, there are spindle shaped cells (fibroblasts – fibrosis, suspected) and accumulation of loose eosinophilic fibrillary material (collagen). Within this there are clear spaces containing 50-150µm wide, loose aggregates of pale basophilic cotton-like material. In the superficial dermis, all the hair follicles are small to non-existent (follicular atrophy, suspected). The overlaying epidermis is approximately 5 cells thick (mild, focally extensive, epidermal hyperplasia), and has a thick stratum corneum composed of several, parallel layers of keratin (lamellar hyperkeratosis).
In areas of skin adjacent to the above described lesion (at the base of the pinna), the superficial dermis is diffusely infiltrated by small numbers of lymphocytes and fewer eosinophils. In the panniculus and subcutaneous muscle associated, there is mostly perivascular infiltration by lymphocytes, with very rare mast cells.
Contributor’s Morphologic Diagnoses:
Severe, focally extensive, granulomatous dermatitis with fibrosis, superficial oedema, epidermal hyperkeratosis and basophilic bacteria – haired skin, pinna
Contributor’s Comment: This is the first red squirrel that was diagnosed with squirrel leprosy a novel disease of red squirrels that has only been detected in the UK so far.7.
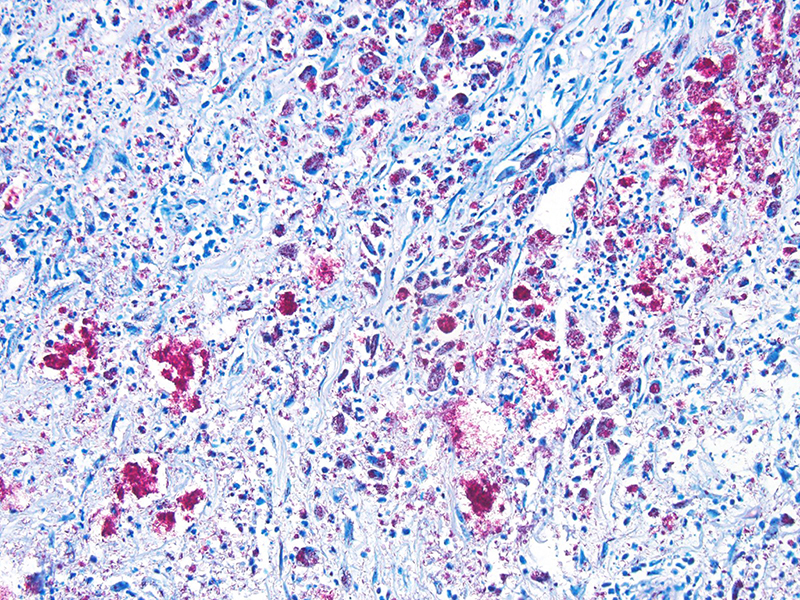
In this case, the diagnosis of mycobacterial disease was first confirmed by Ziehl-Neelsen staining, which revealed intrahistiocytic and intraneural acid-fast bacteria. The role of the gram-positive filamentous bacteria noted within the pinnal lesions was interpreted at first either as a co-infection, or as a secondary infection. Since this first diagnosis, we have recorded the presence of filamentous bacteria in some -but not all- further cases of squirrel leprosy, frequently associated with ulceration of the lepromatous lesions. This suggests that the primary etiological agent are the acid-fast bacteria. Work on the characterization of this sporadic association, as well as the taxonomical identity of these filamentous bacteria is pending.
Following this diagnosis, further sporadic red squirrel cases with similar gross lesions were submitted for examination from diverse areas of Scotland, and we were able to collect six cases from 2006 to 2013. Three of these cases were lost to follow up due to freezer break down, and the gross and histological examination was followed by PCR analyses for the three remaining squirrels (including this case). Briefly, fresh and fixed samples from these were analyzed by PCR for the detection of IS900 and F57 (specific for Mycobacterium avium subspecies paratuberculosis [MAP]), IS901 (specific for MAP), and the gene encoding heat shock protein 65 (hsp65=vgg – present in all Mycobacterium spp). Only hsp65 yielded positive results, and of the amplicons revealed 99% sequence homology with Mycobacterium lepromatosis (FJ924). This was an unexpected finding, as M. lepromatosis had only been reported in human leprosy patients in Mexico from 2008,3 and since then in other human cases from additional locations (e.g. Costa Rica and Singapore).2 There are no human cases of M. lepromatosis reported in the United Kingdom, or any report of M. lepromatosis in other non-human mammal worldwide.
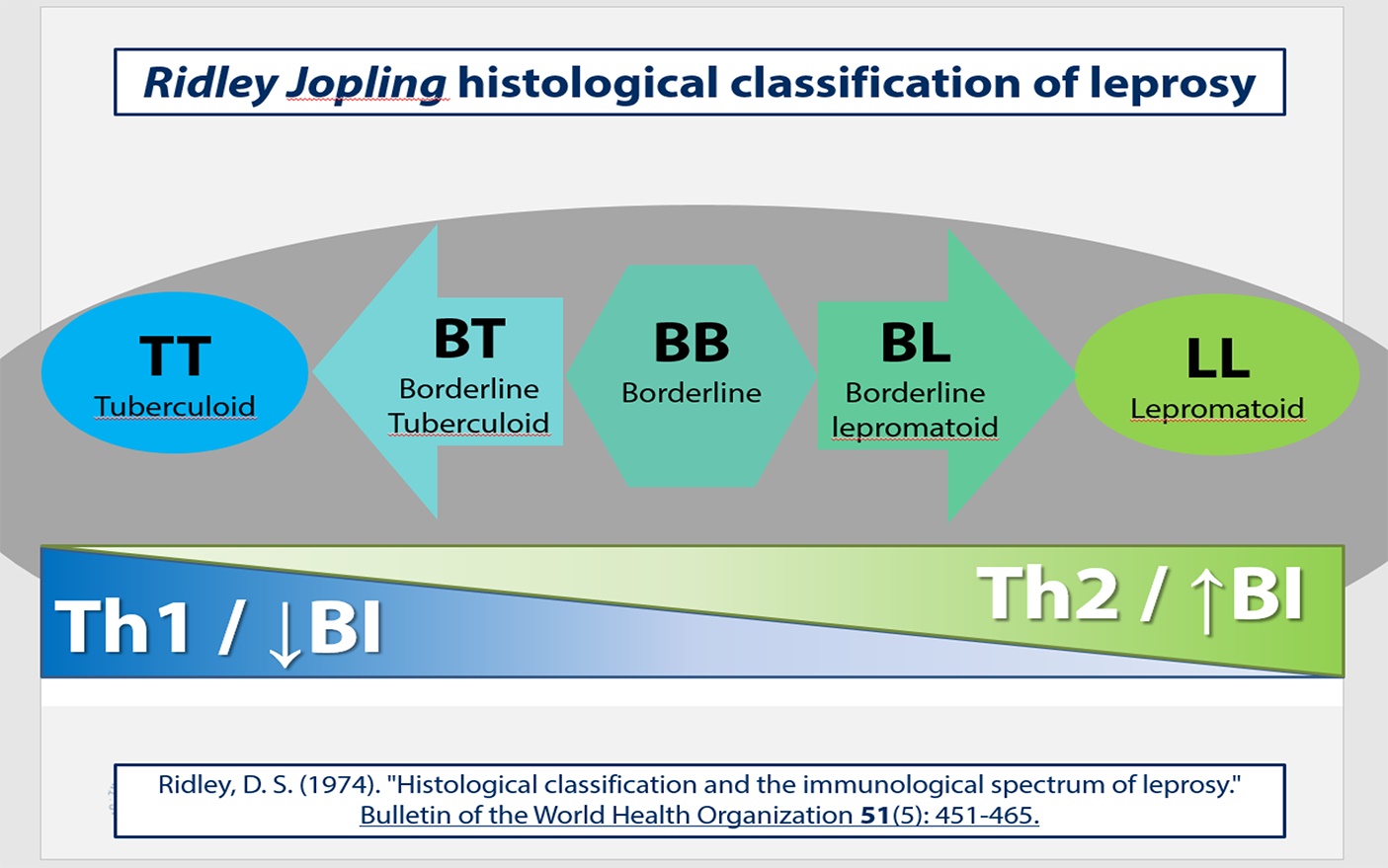
The first report of human leprosy by M. lepromatosis in 2008 has expanded the range of etiological agents of human leprosy to two bacterial species (previously only Mycobacterium leprae was known to cause human leprosy). Human infection with M. lepromatosis is associated with diffuse leprosy, a severe form of leprosy with spreading lesions containing foamy macrophages laden with large numbers of intracytoplasmic acid fast bacteria. The histological presentation we saw in these squirrels is consistent with lepromatous leprosy. The spectrum of human leprosy lesions as described for Mycobacterium leprae is summarized in figure 6. At the extremes of this spectrum M. leprae causes two distinct histological patterns5:
- Tuberculoid leprosy, with dry, insensitive, scaly cutaneous lesions, which histologically feature asymmetric involvement of large peripheral nerves, which are enclosed within granulomas containing low numbers of intracellular acid fast bacilli (paucibacillary). This is associated with a Th1 response, with production of IL-2 and IFN-γ.
- Lepromatous leprosy, with symmetric skin thickening and nodules in distal areas (earlobes, feet). This presentation involves widespread nervous invasion of nerves by Mycobacteria, which are found within Schwann cells, endoneural and perineural macrophages with minimal inflammation. The lesions are composed of large, dermal aggregates of lipid-laden macrophages containing large numbers of acid-fast bacteria (multibacillary). Other organs that may be involved are regional lymph nodes, spleen, liver and testicles. This presentation is also known as anergic leprosy, because of immune unresponsiveness, and features a weak Th1 response and relative increase in Th2 response.
The spectrum contain intermediate categories between the two poles, and is described by the Ridley-Joplin classification scheme.7 This scheme also includes early indeterminate leprosy (IL – usually early lesions that may evolve to one or another pole). The entire scale includes polar tuberculoid leprosy (TT), borderline tuberculoid leprosy (BT), mid-borderline leprosy (BB), borderline lepromatous leprosy (BL), and polar lepromatous leprosy (LL). A further layer of information is provided by the determination of the number of acid-fast bacilli present in the lesions, which is expressed on a 1 to 6 score based on a logarithmic scale called the bacteriologic index (BI). At the extremes are score 1= 1-10bacilli in 100 oil immersion fields (paucibacillary) to score 6= ≥1000 bacilli in 1 oil immersion field (multibacillary).
An important diagnostic feature of leprosy, is the presence of neural lesions, as noted in the red squirrel cases. Intraneural acid fast bacteria are a diagnostic feature, and these may be associated with neuritis or perineuritis.5 Neural lesions are present throughout the spectrum of leprosy lesions, and lead to skin anaesthesia and muscular atrophy that render affected areas susceptible to trauma-associated lesions. It is possible to hypothesize that the filamentous bacteria noted in red squirrels may have entered the skin lesion after trauma, as their presence is associated with ulceration.
Other patterns may be associated with human leprosy, which have not been reported in red squirrels. The Type I reaction, or reversal reaction, involves an increase in cell-mediated immunity in lesions at the borderline part of the spectrum. This leads to progression towards the tuberculoid pole, but also to reactivation of the lesions. This reactivation in results in pain, the appearance of new lesions, erythema/oedema of old lesions, and nerve tenderness and swelling. The type II reaction, or erythema nodosum, is a type III hypersensitivity that occurs on patients with lepromatoid leprosy. These patients develop neutrophilic infiltration of the lesions, oedema, vasculitis/panniculitis, and visceral manifestations.
Raised awareness as a result of the work on Scottish squirrels infected with M. lepromatosis led to the detection of a cluster of similar cases in Brownsea Island, a small island off the south coast of England. Strikingly, sampling and PCR analysis of these cases revealed infection of 25 red squirrels with M. leprae1. This expands the range of possible M. leprae hosts previously, which were humans and nine-banded armadillos.9 Another isolated case of M. lepromatosis was detected in Ireland as part of this further work. The gross and microscopic lesions are very similar in red squirrels infected with M. leprae or M. lepromatosis (indistinguishable in the sample set available).
Further mycobacterial whole genome analysis was possible using enriched techniques (e.g. host DNA depletion tools) on tissues from all the squirrel samples.1 This enabled whole genome sequencing of these bacteria, and phylogenetic comparisons using Bayesian statistics. Comparison of British and Irish M. lepromatosis with two Mexican strains from humans show that they diverged from a common ancestor around 27,000 years ago, whereas the M. leprae strain is closest to one that circulated in Medieval England. Furthermore, comparison between the English and Irish M. lepromatosis isolates revealed divergence approximately 300 years ago, coinciding with the reintroduction of red squirrels in Ireland from British stocks.
Contributing Institution:
Easter Bush Pathology, College of Medicine and Veterinary Medicine, University of Edinburgh, UK (http://www.ed.ac.uk/vet/services/easter-bush-pathology).
JPC Diagnosis:
- Ear, pinna: Dermatitis and neuritis, granulomatous, multifocal to coalescing, severe, with numerous intracytoplasmic bacilli.
- Ear, pinna: Dermatitis, superficial, hyperkeratotic, multifocal to coalescing, moderate, with rare arthropods (acariasis).
JPC Comment: The contributor has provided an outstanding write up on this emerging disease a unique species as well as a fine review on the various types of leprosy, allowing us to examine the history of one of the world’s oldest scourges.
In 2005, genetic analysis was performed on the incredibly stable genome of MA in order to map its origins.6 Incredibly, a cases of leprosy throughout history are derived from a single clone throughout history, and very rare polymorphisms in single nucleotides allow for insight into the disease’s roots. The disease originated in Eastern Africa or the Near East, and spread into Asia and Europe with progressive human migrations, often accompanying patterns of colonization and the slave trade. Leprosy was introduced into the Americas with the advent of slavery, either by Europeans or Western Africans.
Sadly, no disease has resulted in more transiently or social stigmata than leprosy. The etymology of the word “leprosy” is derived from Greek or from old English, meaning (scaly skin). While it is difficult to make retrospective diagnoses from symptoms of diseases in ancient writings, lesions strongly suggestive of leprosy known as “tzaraath” (and reputed to be healed by Jesus in the New Testament) are found in the Bible and the Talmud, and similar lesions are described in the Feng Zhen Shi (266-246 B.C., China). In 2009, a 4000-year old skeleton was uncovered in India demonstrating patterned lesions of leprosy.
The stigmata associated with leprosy began in the Middle Ages – afflicted individuals were often required to wear bells on their persons to announce their passage in the streets and literally thousands of leprosariums sheltered the infected (as well as many others with other diseases whose only affront was being a family member of an infected individual or simply indigent. Mandatory segregation of inflicted individuals was practice in a number of countries (with Japan in 1996 being the final country to rescind this stigmatizing law) and many countries (including the UK) made it a practice to segregate patients by gender in leprosaria, in the unfounded belief that children arising from two infected individuals would be born with the disease. Some of the most restrictive practices were the norm in India which in the 1900s regionally prohibited infected individuals from traveling by train, diving, running in local elections and even made it a legal justification for divorce. Some of these laws, written prior to the development of successful multidrug therapy and the Indian healthcare’s systems very successful campaign against the diseases, still remain on the books.
In 1873, Dr. G.H Armauer Hansen discovered the causative agent, making leprosy the first disease to be recognized as being caused by a bacterium. The disease today is often referred to as “Hansen’s disease”, a term preferred by patients over the term “leper”. In the 1940’s the sulfone drug promin became the first effecting therapy. Today, multidrug therapy of rifampicin, clofazimine, and dapsone (a derivative of promin) are highly affected in treating leprosy.
Due to effectiveness of multidrug therapies, the sole remaining leprosarium in the US in Carville, Louisiana was closed in 1999 in favor of outpatient treatment of those afflicted with Hansen’s disease. Approximately 200 cases are diagnosed yearly in the U.S, and treated effectively if the disease is recognized early. Interestingly, zoonotic leprosy, transmitted from armadillos to humans has been confirmed in parts of the southern U.S.9
In this case, the amount of hyperkeratosis and superficial inflammation seemed excessive for mycobacteriosis. In the electronic disc provided with this series, there are rare cross sections of a chitinous exoskeleton, suggestive of an arthropod, overlying the epidermis. On serial sections of the slide provided for special stains, multiple intact arthropods were identified within regions of hyperkeratosis. This is consistent with acariasis in addition to leprosy in this animal.
A recent publication on “atypical histiocytosis” in red squirrels described animals with similar gross and histologic lesions to those in this case, but IHC, special stains, and PCR were negative for Mycobacterium spp.8
Following the review of the case, the moderator discussed the widespread distribution of mycobacteria in a number of other species including fish, reptiles and amphibians. Other species of note for mycobacterial infections include elephants, which are often infected by M. tuberculosis, noting that it may is advantageous to wear personal protective gear when performing necropsies on elephants. She also noted the prevalence and severity of mycobacterial in sygnathid fish, including sea horses, sea dragons, and pipefish.
References:
- Avanzi C, del-Pozo J, Benjak A, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science. 354(6313) 2016: 744-747.
- Han XY, Aung FM, Choon SE, Werner B. Analysis of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis in four countries. Am J Clin Pathol. 142(4) 2014: 524-532.
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 130(6) 2008: 856-864.
- LaRose JP, Meredith AL, Everest DJ, et al. Epidemiological and postmortem findings in 262 red squirrels (Sciurus vulgaris) in Scotland, 2005 to 2009. Vet Rec. 167(8) 2010: 297-302.
- McAdam AJ, Sharpe AH. Leprosy. In: Kumar V, Abbas AK, Fausto N, Aster JC, ed. Robbins & Cotran pathologic basis of disease. 8th ed. Philadelphia, US: Saunders Elsevier; 2010:372-373
- Monot M, Honore N, Darnier T, Araoz R, Coppee JY, Lacroix C, Sow S. On the origin of leprosy. Science; 2005, 308:1040-1042.
- Meredith A, Pozo JD, Smith S, et al. Leprosy in red squirrels in Scotland. Veterinary Record. 175(11) 2014: 285-286.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 51(5) 1974: 451-465.
- Smith SH, Stevenson K, del-Pozo J, Moss S, Meredith. Atypical histiocytosis in red squirrels (Sciurus vulgaris). J Comp Path 2017; 156:446-450.
- Truman RW, Singh P, Sharma R, et al. Probable Zoonotic Leprosy in the Southern United States. New England Journal of Medicine. 364(17) 2011: 1626-1633.