Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 10
28 November 2018
CASE III: 11136 (JPC 4066393).
Signalment: 21-year-old male Indonesian origin cynomolgus macaque (Macaca fascicularis).
History: Following sedation for an experimental procedure two weeks prior to death, this female macaque lost 6% of its body weight. It had a 4 year history of eating an atherogenic diet and was in obese body condition. The day prior to death, the animal presented with lethargy, dehydration and hypothermia (94.4?F). Following an unsuccessful attempt at supportive care, the animal died and was submitted for necropsy.
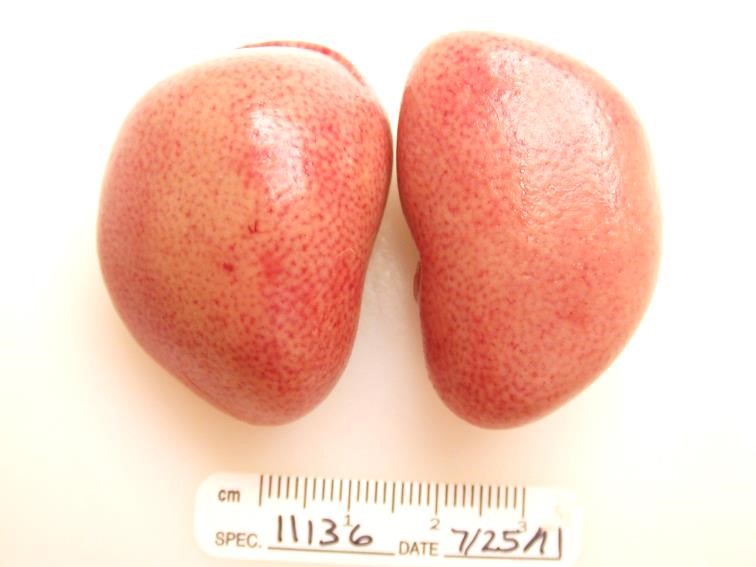
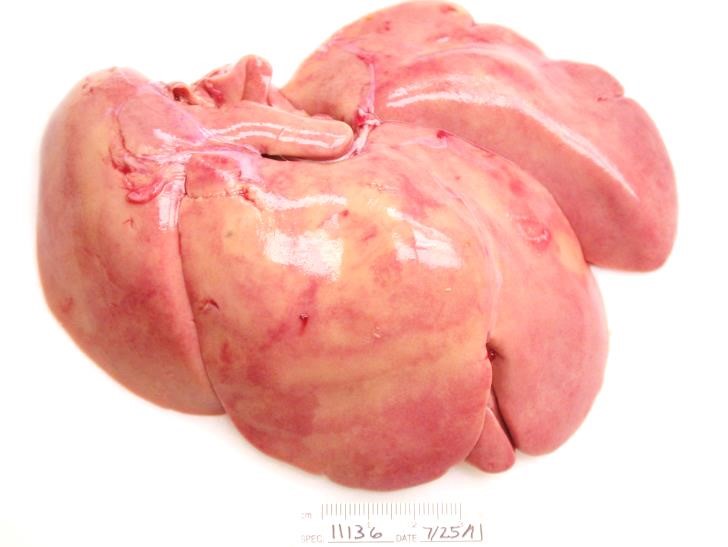
Gross Pathology: The animal was obese with abundant visceral and subcutaneous adipose tissue. The kidneys were diffusely tan. The liver was diffusely pale yellow with a slight reticular pattern, had slightly rounded edges, and cut sections floated in formalin. The luminal surface of the aortic intima was irregularly thickened by pale yellow to tan plaques (atherosclerosis). The stomach and small intestine contained only clear mucinous fluid, flecked with ingesta.
Laboratory results: Blood chemistry values acquired the day prior to death revealed marked azotemia (BUN 107mg/dL; creatinine 8.4mg/dL) with hyperphosphatemia (13.60mg/dL), mildly elevated ALP (239U/L), and moderate hypoproteinemia (total protein 5g/dL; albumen 2.1g/dL).
Microscopic Description:
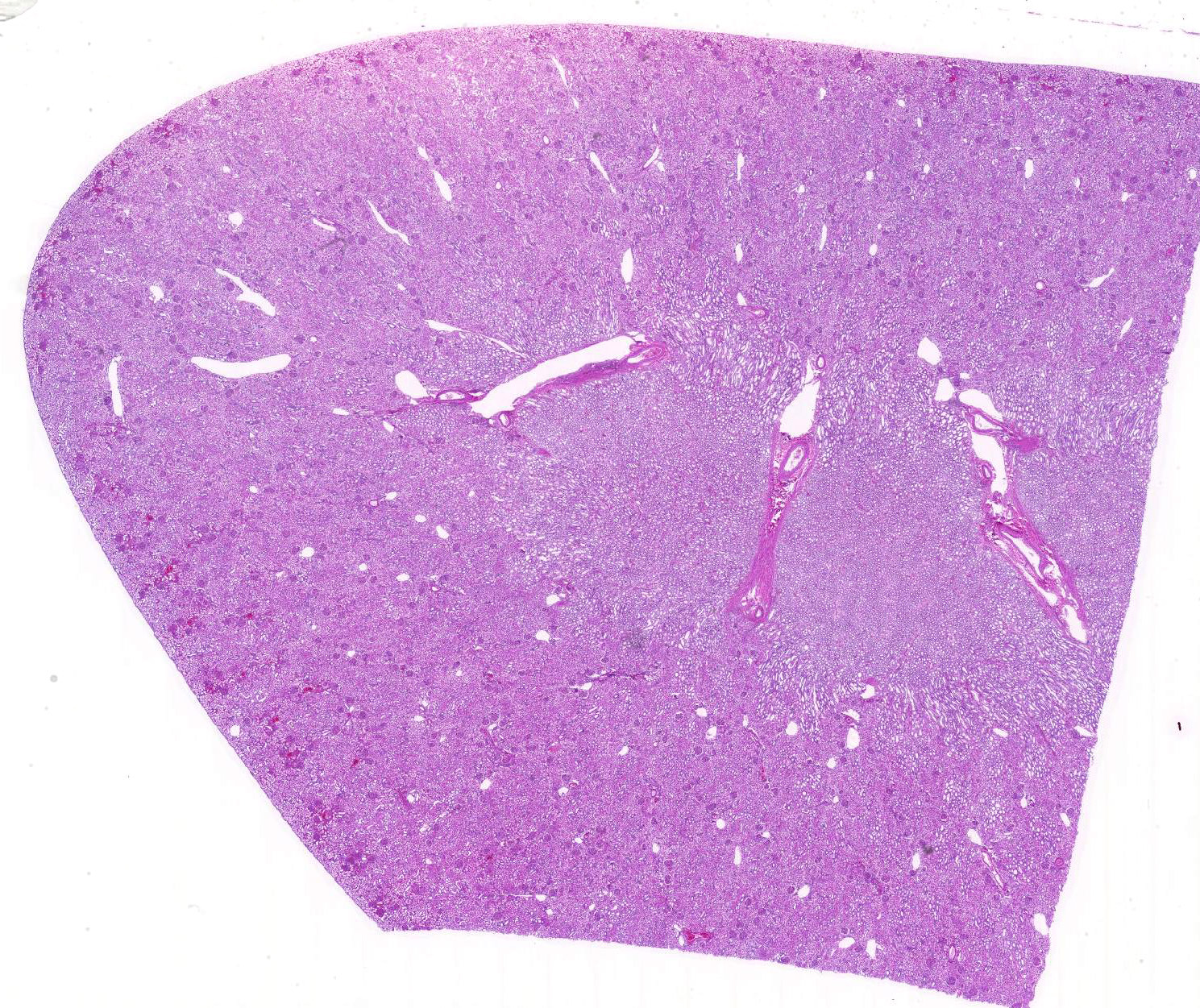
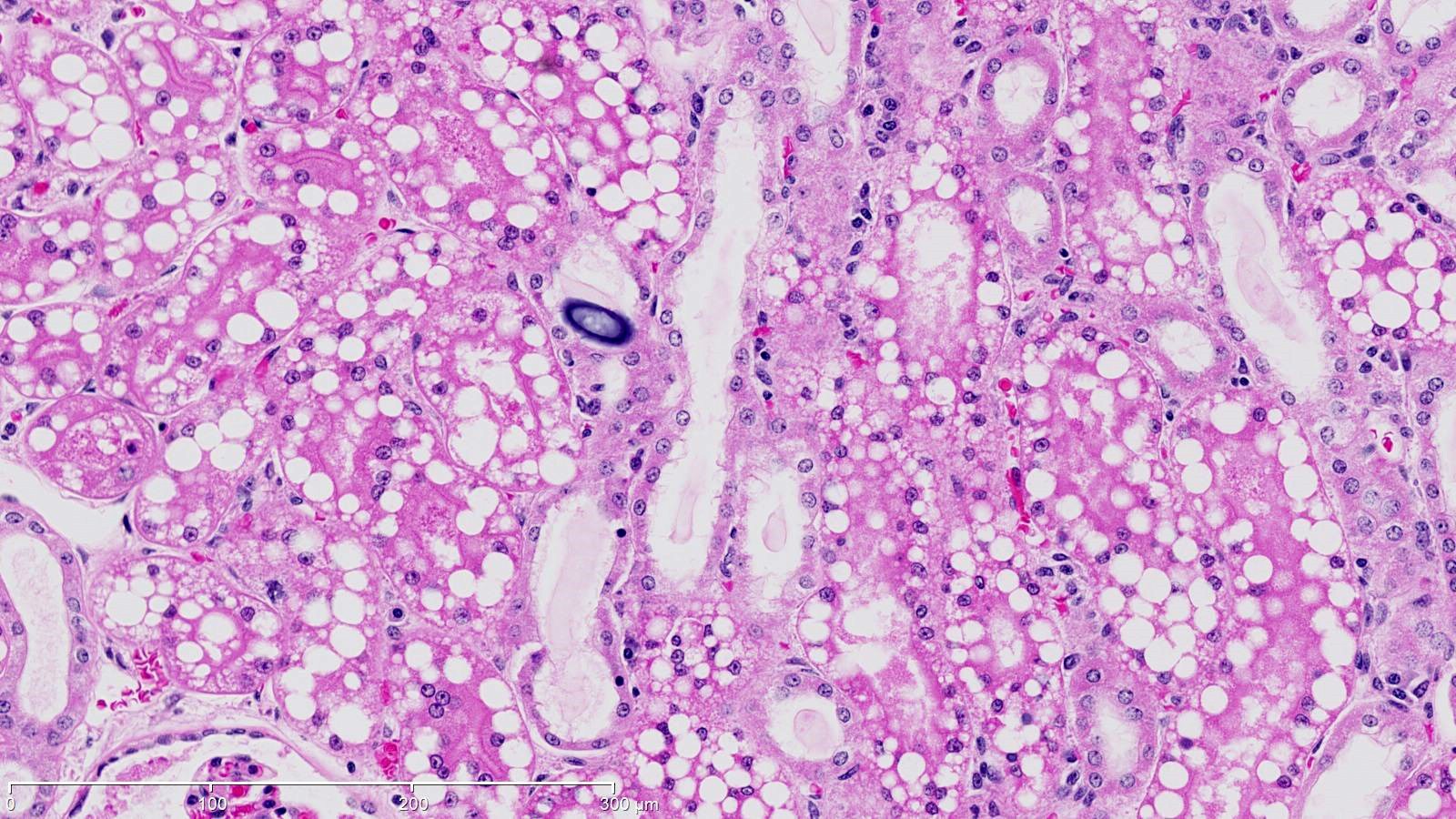
The renal cortical proximal tubular epithelial cells were markedly dilated by variably-sized clear cytoplasmic vacuoles (lipid), up to 40 microns in diameter. Tubular lumina were often reduced by these plump cells and occasionally contained neutrophils or sloughed epithelial cells. The epithelial cells in some tubules were attenuated and widely spaced indicative of epithelial loss. Scattered tubules contained round lamellar basophilic material (mineral). A few glomeruli had thickened basement membranes and, rarely, glomeruli were atrophic.
Contributor’s Morphologic Diagnoses:
Kidney: Vacuolar degeneration, diffuse, marked with epithelial loss and intraluminal mineral, renal tubular epithelium (renal lipidosis)
Kidney: Glomerulonephritis, diffuse, chronic, minimal
Contributor’s Comment: Additional relevant microscopic findings included vacuolar hepatocellular degeneration (hepatic lipidosis) and necrosis of adipose tissue. Death was attributed to acute renal failure due to the massive accumulation of lipid and the associated metabolic derangements, all consistent with fatal fasting syndrome of obese macaques. The necropsy findings, along with the marked azotemia, hyperphosphatemia, advanced age, recent stressors and obesity with rapid weight loss are all consistent with this syndrome.8
Fatal fasting syndrome has been reported in African green monkeys (Cercopithecus aethiops), cynomolgus macaques (Macaca fascicularis) and rhesus macaques (Macaca mulatta), and shares similarities with conditions in other species, including hyperlipidemia in Shetland ponies, hepatic lipidosis in cats and guinea pigs, and pregnancy toxemia in ruminants.2 Obese, female, aged macaques are particularly susceptible to this syndrome, which is often precipitated by a period of anorexia, weight loss, or stress.2,3,7,8 The pathogenesis has not yet been fully elucidated, but a period of inadequate caloric intake in obese animals is a common theme among the disease profiles of the all the aforementioned species. Regardless of the initiating factor, an over-compensation of the liver results in fatty acid mobilization beyond the body’s ability to metabolize, resulting in a metabolic imbalance with an inability to process the excessive triglycerides and lipoproteins.4,7
The most common associated clinical pathology finding is renal azotemia.8 Hypertriglyceridemia is a common associated finding among the similar metabolic derangements across species. Triglycerides are not considered inherently toxic, but the products produced with triglyceride breakdown or failure of esterification (non-esterified fatty acids, ceramides, diacylglycerols) have detrimental effects on cells.10 Lipotoxicity has been described in a several animal models throughout non-adipose tissues including cardiac and skeletal myocytes, hepatocytes, pancreatic B-cells and renal tubules.9 Lipotoxic mechanisms leading to cellular dysfunction and injury may involve reactive oxygen species, intra-cellular pathway disruption, organellar damage, and lipid-induced apoptosis.1 Renal tubular degeneration and detachment can lead to luminal tubule obstruction, increasing the interstitial pressure and ultimately reducing the glomerular filtration rate with subsequent acute renal failure.4
The reason for the female predisposition to this syndrome is unclear, but hyperlipidemia in Shetland ponies is similarly gender predisposed.5 In cats with hepatic lipidosis, males and females are equally represented.6
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology/Comparative Medicine
Medical Center Boulevard
Winston Salem, NC 27157-1040
http://www.wakehealth.edu/School/Comparative-Medicine/Training-Programs/ACVP.htm
JPC Diagnosis: Kidney, proximal convoluted tubular epithelium: Lipidosis, diffuse, severe with mild tubular proteinosis.
JPC Comment: The contributor has done an excellent job describing this classic metabolic condition in the macaque.
One of the obvious morphologic abnormalities in this section is the disproportionate amount of lipid within the cells of the proximal convoluted tubules. As the proximal convoluted tubules are the site of albumin resorption in the kidney, and free fatty acids (FSA) are normally complexed to albumin1, this may explain the predominance of lipid within cells of the proximal convoluted tubules. Whether significant lipid synthesis from non-lipid substrates occurs in renal tubular epithelium and pathways of lipid export from the kidney are subjects of current conjecture and research.1
The link between lipidosis and kidney disease was first suggested more than 150 years ago by Rudolph Virchow, and the association between kidney disease and renal lipid accumulation has been demonstrated in the number of rodent models including mice on high fat diets, mice and rats with leptin deficiency, Type 1 diabetes and various transgenic models.1 Lipotoxicity is usually accompanied by accumulation of neutral lipids in renal tubular epithelium and other cells as triglycerides. Triglycerides in themselves are non-toxic, with toxicity deriving mainly from long-chain non-esterified fatty acids (NEFA) and other breakdown products such as ceramides and diglycerols, and the association with NEFA-induced mitochondrial de-energization or persistent ATP depletion has been well-documented in renal reperfusion injury as well other forms of lipid-induced nephrotoxicity.2
Fatal fasting syndrome in macaques present in numbers of similarities to syndromes associated with stress, anorexia and lipidosis of multiple organs in both the cat and the horse. Hepatic lipidosis is a well-known metabolic condition in cats resulting from severe prolonged caloric and protein restriction. Carbohydrate restriction is the primary driver of mobilization of peripheral fat stores and flooding of the liver with NEFA. In the normal state, NEFA enter the mitochondria and are converted to energy. In excess, NEFA are either stored within the hepatocyte, or esterified with lipoproteins and re-secreted as VLDLs. In cats with hepatic lipidosis, concurrent protein starvation precludes production of lipoproteins and retention of massive amounts of triglycerides as lipid vacuoles within the hepatocyte.
In horses, hyperlipidemia is associated with additional risk factors including breed (ponies, donkeys, and miniature breeds), gender (with mares accounting for between 75 and 100% of cases) and stress (more commonly associated with late pregnancy and early lactation). In ponies and donkeys, hyperlipidemias usually a primary disease process with stress and obesity appearing to be particularly important predisposing factors. Gross and histologic findings mirror similar syndromes in macaques and cats with gross lipidosis of multiple organs - hepatic lipidosis in affected animals may be severe enough to result in signs of colic from overstretching of the hepatic capsule.5
On a peripherally related subject, a recent article identified interstitial lipid accumulation as a long-term histologic findings in cats with chronic renal disease.8 Data from the study suggests that the interstitial lipid accumulation may be the result of tubular epithelial degeneration and lysis as well as tubular basement membrane fragmentation in a species in which tubular lipidosis is often seen in the normal state. In the animals of the study, interstitial lipid was not equally distributed between the right and left kidneys, but all was found in the cortical regions. In light of recent research on mechanisms of renal lipoma toxicity, this is an interesting finding which may contribute to the overall progression of this common disease in cats.8
Within the seminar, there was discussion about the use of the term “vacuolar degeneration, lipid-type” versus “lipidosis” in the morphologic diagnosis for this particular lesion. There was general agreement that the former is better reserved for situations in which morphologic evidence of cellular degeneration is noted beyond simple lipid vacuolation or swelling. In this case, without either clinicopathologic evidence of tubular damage, or morphologic evidence of tubular damage, it was thought that lipidosis (or vacuolar change) would be most a more appropriate choice..
References:
- Bobulescu, Ion Alexandru. "Renal lipid metabolism and lipotoxicity." Curr Opin in Nephr Hypertens 4 (2010): 393.
- Bronson RT, O’Connell M, Klepper-Kilgore N, Chalifoux LV, Sehgal P: Fatal fasting syndrome of obese macaques. Lab Anim Sci 32(2):187-191, 1982
- Christie KL, Valverde CR: The use of a percutaneous endoscopic gastrotomy (PEG) tube to reverse fatal fasting syndrome in a cynomolgus macaque (Macaca fascicularis). Contemp Topics 38(4):12-15, 1999
- Cotran RS, Kumar V, Collins T: Cellular pathology II: adaptations, intracellular accumulations, and cell aging. In: Robbins’ Pathologic Basis of Disease, 6th ed., pp. 38-40, 993-995. WB Saunders, Co., Philadelphia, PA, 1999
- Hughes, K. J., D. R. Hodgson, and A. J. Dart. "Equine hyperlipaemia: a review." Aust Vet J3 (2004): 136-142.
- Kelly WR: The liver and biliary system. In: Pathology of Domestic Animals, ed. Jubb KVF, Kennedy PC, Palmer N, 4th ed., vol. 2, pp. 335-336. Academic Press, Inc., New York, NY, 1993
- Laber-Laird KE, Jokinen MP, Lehner NDM: Fatal fasting liver-kidney syndrome in obese monkeys. Lab Anim Sci 37(2):205-209, 1987
- Lewis AD, Colgin L: Pathology of Noninfectious Diseases of the Laboratory Primate. In: The Laboratory Primate: Wolfe-Coote, Sonia. pp. 65-66. Academic Press, 2005.
- Martino-Costa AL, Malhao F, Lopes C, Dias-Pereira P. Renal interstitial lipid accumulation in cats with chronic kidney disease. J Comp Path 2017; 157:75-79.
- Weinberg JM. Lipotoxicity. Kidney Int 70: 1560–1566, 2006.