CASE III: MS19-3845/MS19-3861 (JPC 4136807)
Signalment:
6 week old and 5 month old, male, C57Bl/6 mice
History:
Two mice were infected IP with 1,000 blood trypomastigotes of Trypanosoma cruzi. A month later, one mouse was euthanized when it developed clinical signs and the other was found dead.
Gross Pathology:
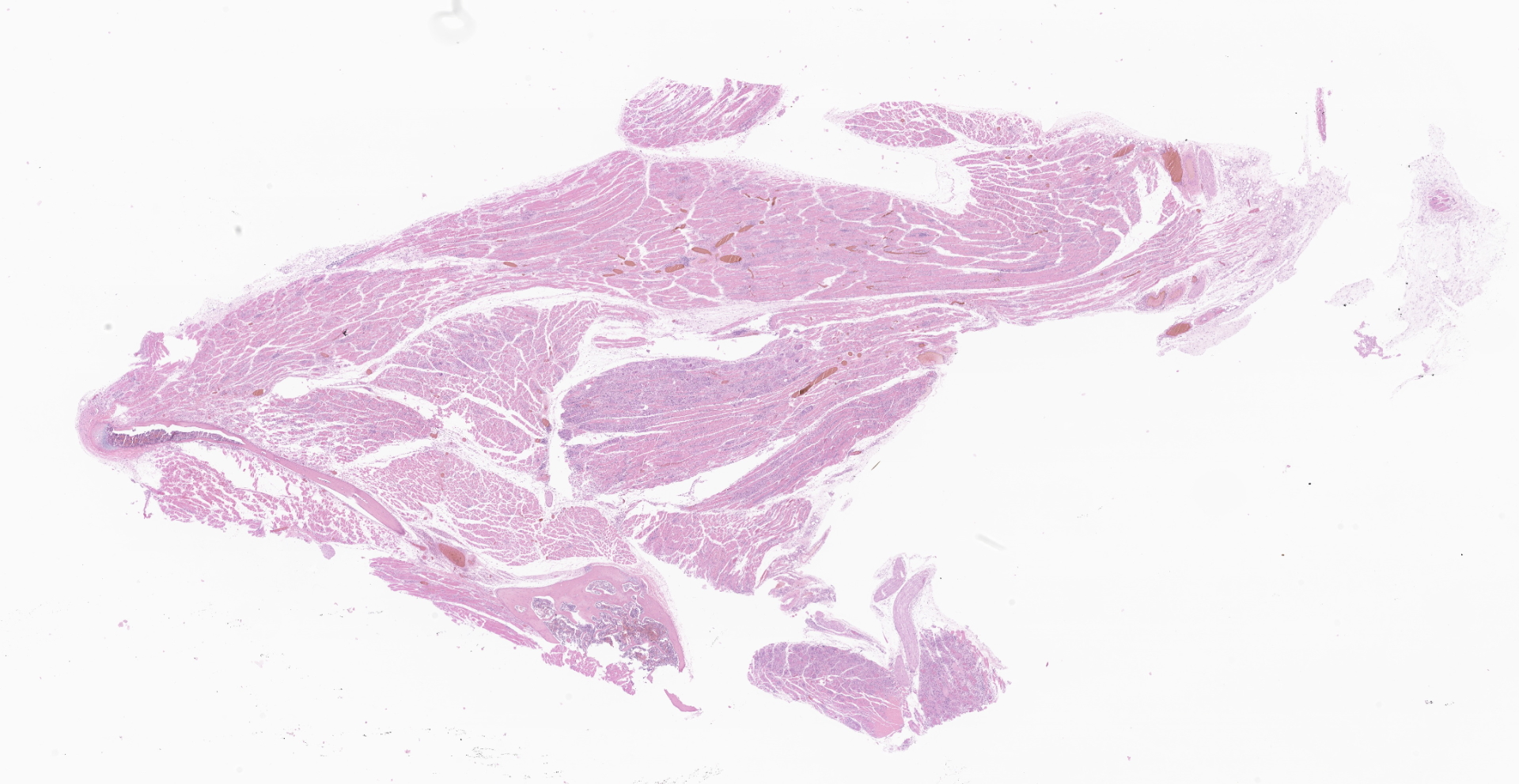
There was pale tan streaking of the semimembranosus, semitendinosus, and portions of the biceps femoris on both hind legs. The iliopsoas and the hypaxial muscles of the lumbar region also had pale streaking.
No other lesions were present.
Laboratory Results:
None submitted.
Microscopic Description:
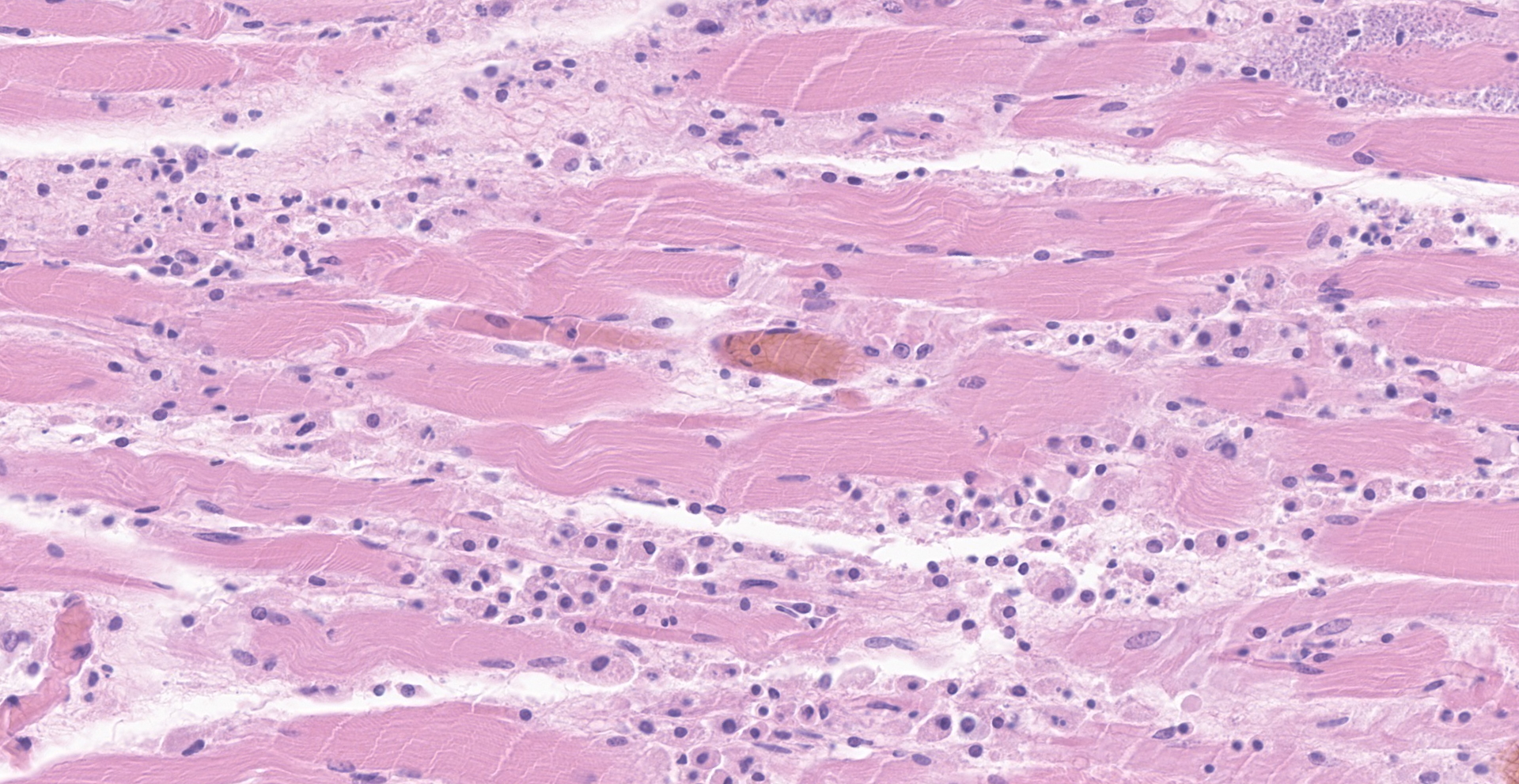
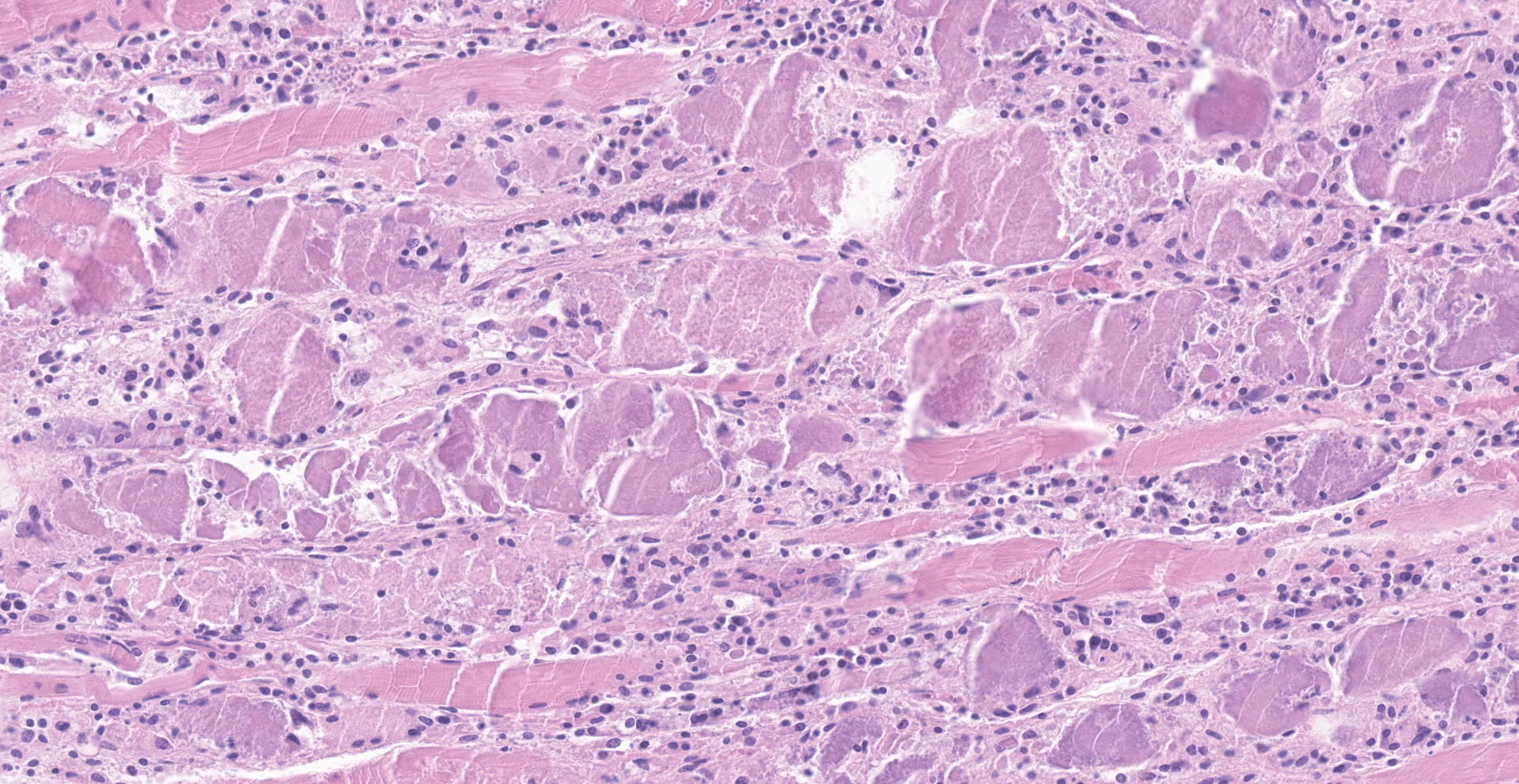
Skeletal muscle: There are multifocal ? coalescing areas where muscle has undergone one of the following changes: loss of striations, hypereosinophilia, fragmentation, necrosis and mineralization. Within some muscle fibers, there are large, up to 250um, pseudocysts containing numerous amastigotes. Amastigotes are round, 2-4um with a central nucleus and adjacent kinetoplast. In some areas, the amastigotes appear to be transforming into trypomastigotes. Between the degenerating and necrotic muscle fibers, there are numerous macrophages with fewer lymphocytes, neutrophils, and plasma cells. Regeneration was not seen.
Other lesions:
Heart: myocarditis, moderate with amastigotes
Right atrium: multiple thrombi
Pancreas: acinar loss, moderate
Spleen: plasmacytosis, mild
Contributor's Morphologic Diagnoses:
Skeletal muscle: myositis, multifocal and focally extensive, severe with amastigotes and trypomastigotes.
Contributor's Comment:
Chagas disease or American trypanosomiasis is caused by the flagellate protozoan Trypanosoma cruzi. Transmission is by triatomine insects (Triatoma infestans, Rhodnius prolixus, Triatoma dimidiata and Parastrongylus), also known as assassin or kissing bugs.5 Infection cycles between human, wild, and domestic animal hosts.1,2 The primary route of infection is by bug bite (70%). Less common modes of transmission include congenital (26%) and, accounting for less than 1% of cases, transfusion/organ transplantation, sexual, and laboratory accident.4,8 Outbreaks have also been caused by ingestion of contaminated food.3,4
Of those who are infected, 70-80% never develop clinical disease. The other 20-30% progress over years to develop chronic disease in which they commonly develop cardiomyopathy. A small percentage (10%) develop gastrointestinal disease with or without associated cardiomyopathy.3,4
Bugs become infected while taking a blood meal and ingesting blood trypomastigotes. The trypomastigotes mature in the midgut becoming epimastigotes and then, as they pass through the hindgut, develop into infective trypomastigotes. The trypomastigotes are then deposited with feces as the bug takes a second meal. Infection occurs when feces are rubbed into broken skin or mucosa. At the inoculation site, trypomastigotes infect nucleated cells via fusion of host cell lysozymes9, transform into amastigotes, divide by binary fission, develop flagella, and then are released as infective trypomastigotes back into the blood to travel to other tissues or infect local cells. Infection by feces (Sterocorarian transmission) is inefficient and is estimated to be between 1-4% per year.3
Chagas disease can be separated into acute, indeterminate, and chronic phases.
Acute phase: After being infected, symptoms may not develop but, if they do, they are most often nonspecific.5 Rarely, after a 1-2 week incubation period, there may be swelling around the wound the site (Chagoma) or of the eye (Romana's sign).3 Biopsy samples from a Chagoma have intrahistiocytic amastigotes and lymphocytes. Swelling around the eyelid is due to edema and conjunctivitis. A morbilliform rash (schizotrypanides) may also develop. Reported microscopic changes are nonspecific, vascular dilation, edema with perivascular lymphoplasmacytic aggregates. Parasites have been reported not to be associated with the rash.8
During the acute phase, approximately 1%, of those infected (mostly young infants) develop acute lymphadenopathy, hepatosplenomegaly, encephalitis, myocarditis and heart failure.3,4
The acute phase lasts approximately 4-8 weeks and then disappears presumably due to a cell mediated response.3 Eosinophilia is not reported to be associated with the initial immune response.8 During this time, infection can be detected by seeing the organisms in a Giemsa-stained blood or CSF smear and PCR and culture.3
Indeterminant phase: This phase lasts years or for the rest of an individual's lifetime. Although they are infected, there are no clinical signs. Parasites are not found in blood smears. PCR and serological results are variable. Infection may become reactivated in immunosuppressed individuals who then go on to develop acute disease with fever, erythematous nodules/plaques, myocarditis and encephalitis associated with numerous amastigotes.3,4
Chronic phase: This phase develops after years of being infected. Clinical presentation may include one or more of the following: cardiac conduction abnormalities, dilated cardiomyopathy, left ventricular aneurysm, and congestive heart failure. Thromboembolism and stroke may develop secondarily.3,4
Early myocardial changes include degeneration and necrosis with infiltration primarily of macrophages and neutrophils and, with more chronic lesions, T-cells, eosinophils and plasma cells. Early inflammatory and myocyte changes may be replaced by mild fibrosis, myocyte hypertrophy and minimal ? mild inflammation. More chronic lesions have focally extensive muscle atrophy and loss with thick, transmyocardial fibrosis. Often, in this chronic phase, there may be no inflammation and very few or no parasites. Even by PCR, the organisms are not always detected.4 Repeated serologic and PCR tests may be needed to detect chronic infection.3
Gastrointestinal disease may be seen in 10% of those who are infected whether they have cardiac disease or not. Although megacolon and megaesophagus are the most common gastrointestinal presentations, dilation anywhere along the intestinal tract can occur. Infection of the enteric nervous system results in denervation of the intestines and to dilation.3,4
T. cruzi can infect any nucleated cell in the body and, in the heart, it invades the myocytes, nerves, endothelial cells and adipocytes. Why the organism targets myocytes may be due plasma membrane repair mechanisms which allow for entry of the parasite into the cell.4
The pathogenesis of T. cruzi is incompletely understood but it involves complicated and intertwined mechanisms involving the virulence of the strain of T. cruzi, the route of infection and the number of organisms, re-infection, the unique innate and acquired immune responses of the host, and the persistence of the organism within tissues. Initial responses to infection are T-cell mediated which reduce but do not clear infection. Many immune responses of the host (cytokines, chemokines, reactive oxygen molecules and antibodies) do not kill all parasites and end up enhancing the infectivity of and damages caused by it.1,3 Adipose tissue has been identified as a reservoir for the parasites.4
The World Health Organization has designated Chagas disease as one of the top neglected tropical diseases. Chagas is associated with poverty where housing with cracked walls and thatched roofs allow bugs to live in close proximity to people.10 Since the institution of insecticide and bed net use along with screening of blood donors and pregnant women, the number of people infected has decreased significantly.3 Still, according to the CDC, 8 million people in Mexico, Central and South America are infected. Migration away from these endemic areas has led to individuals, most of whom are not aware that they are infected, to be identified in North America, Europe, Japan, and Australia. In the United States, 300,000 people are believed to be infected. In addition, triatomine insects can be found in most states and any domestic or wild mammal can be infected and serve as a reservoir/host.1,5 In the United States and in other regions where Chagas disease is now found but is not widespread, control strategies are focused on preventing transmission from blood transfusion, organ transplantation, and from mother to baby.5 Currently, blood donors are screened for parasites/antibodies. As of 2017, only six states (Arizona, Arkansas, Louisiana, Mississippi, Tennessee and Texas) have designated Chagas as a reportable disease.2,3
Treatment with benznidazole or nifurtimox may lead to 80 ? 100% cure in newborns and those known to be acutely infected. Benznidazole may decrease the rate of cardiomyopathy progression.3
Currently, there is no vaccine for Chagas. The inability to predict who will progress from the indeterminate phase and develop cardiac disease continues to be problematic. To date, a definitive molecular marker to identify those who will or are developing cardiac disease has yet to be identified although, microRNA-2208a, a plasma marker for cardiac disease, has been suggested.4
Contributing Institution:
National Institutes of Health
Division of Veterinary Resources
Building 14E/119B
Bethesda, MD 20892
starostm@mail.nih.gov
Phone: 301-443-7251
JPC Diagnosis:
Skeletal muscle: Rhabdomyositis, necrotizing and histocytic, multifocal, moderate, with intrasarcoplasmic and extracellular protozoal amastigotes and myofiber mineralization.
JPC Comment:
The contributor provides an outstanding review of Chagas disease, an emerging disease in the southwestern United States that historically was restricted to Central and South America.
Chagas disease far predates the arrival of Europeans in the New World, as T. cruzi kinetoplast DNA has been identified in exhumed Peruvian and Chilean mummies from approximately 7000 BCE, with similar findings in Central America. It is likely humans became part of the sylvatic cycle of Chagas disease as they began populating the South American coast approximately 9500 years ago. Interestingly, the Andeans domesticated rodents for both consumption and ritualistic use, which likely further attracted hematophagous insects such as Triatoma infestans, which are adept at survival in traditional wattle and daub houses.6
Interestingly, Charles Darwin may have unknowing contracted Chagas disease during his famed expedition aboard the HMS Beagle. While visiting in the Chilean port city of Valparaiso in 1834, he became severely ill and was bedridden for 7 weeks for what was thought to be typhoid disease, although no other crew members became ill. In addition, a March 1835 journal entry describes a nocturnal attack of a Reduvid bug as "the most disgusting to feel soft wingless insects about one inch long crawling over one's body; before sucking they are quite thin, but afterwards round and bloated with blood, and in this state they are easily squashed". Additional exposure likely occurred as he studied T. infestans, known at the time as the "great black bug of the Pampas", for at least four months. Darwin reported no additional symptoms consistent with Chagas disease from 1835-1841, which is consistent with the indeterminate phase as described by the contributor. Between 1841-1861, Darwin experienced symptoms consistent with the chronic phase of Chagas disease, including heart palpitations, extreme fatigue, and vomiting. Given his family's history of psychiatric tendencies, physicians believed he was suffering from hypochondriasis. However, Darwin's symptoms persisted and he was later diagnosed with heart failure prior to his death in 1882. Although it is uncertain if Darwin was truly infected with T. cruzi, the history of exposure to a Reduvid bug and progression of clinical signs consistent with the acute, indeterminate, and chronic phases of infection are highly consistent.6
During the early 1900s, a young Dr. Carlos Chagas was one of several Brazilian physicians dispatched to investigate a malaria outbreak delaying construction of the Brazil Central Railroad in the state of Minas Gerais. During his travels, Dr. Chagas was notified of a nocturnal hematophagous insect found to contain a flagellated parasite similar to one he had previously described in monkeys and named Trypanosoma minensis. Samples were sent to his mentor and director of the National Institute of Serum Therapy in Rio, Dr. Osvaldo Cruz, who then inoculated several laboratory monkeys. A month later, Dr. Chagas found the parasite within the blood of the monkeys was different than T. minensis, and named the parasite T. cruzi to honor his mentor. Dr. Chagas proceeded to seek a human host and later found the parasite in the blood of a two-year-old girl named Berenice, who had fever, facial edema, and hepatic-splenic-lymph node syndrome. Dr. Chagas is therefore credited with performing a "reverse triple discovery" by first identifying the vector, followed by the parasite, and finally the host.6
As noted by the contributor, iatrogenic transmission of Chagas disease has been of growing concern, with reports of T. cruzi transmission via kidney transplants in Brazil during the 1980s, with similar reports of accidental transmission occurring in the United States 20 years later. In addition, large numbers of individuals in endemic areas on organ transplant waitlists are infected, including 7% of Argentinians. This is a concern, given the risk of reactivation occurring as the result of transplant related immunosuppression, which is reported to occur in 23-75% of patients. However, treatment with benznidazole for 30-60 days is effective, with only 0.3% mortality associated with Chagas disease reactivation.6
References:
1. Acevedo GR, Girard MC, Gómez KA. The Unsolved Jigsaw Puzzle of the Immune Response in Chagas Disease. Front Immunol. 2018;9:1929.
2. Bennett C, Straily A, Haselow D, et al. Chagas Disease Surveillance Activities - Seven States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(26):738-741.
3. Bern C. Chagas' Disease. N Engl J Med. 2015;373(19):1882.
4. Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM. Pathology and Pathogenesis of Chagas Heart Disease. Annu Rev Pathol. 2019;14:421-447.
5. CDC. American Trypanosomiasis. https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html
6. Chao C, Leone JL, Vigliano CA. Chagas disease: Historic perspective. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5):165689.
7. Gomes C, Almeida AB, Rosa AC, Araujo PF, Teixeira ARL. American trypanosomiasis and Chagas disease: Sexual transmission. Int J Infect Dis. 2019;81:81-84
8. Hemmige V, Tanowitz H, Sethi A. Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. Int J Dermatol. 2012;51(5):501-508.
9. Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388-1402.
10. World Health Organization https://www.who.int/neglected_diseases/2010report/en/