CASE IV: 18042E (JPC 4136503)
Signalment:
Four-year-old, male rhesus macaque (Macaca mulatta)
History:
This animal was exposed to 10.7 Gy whole thorax irradiation using 6 MV linear accelerator-derived photons delivered at a dose rate of 100 cGy/min using methods similar to those previously described.2 The animal was euthanized 63 days post-irradiation due to an elevated respiratory rate (experimental endpoint).
Gross Pathology:
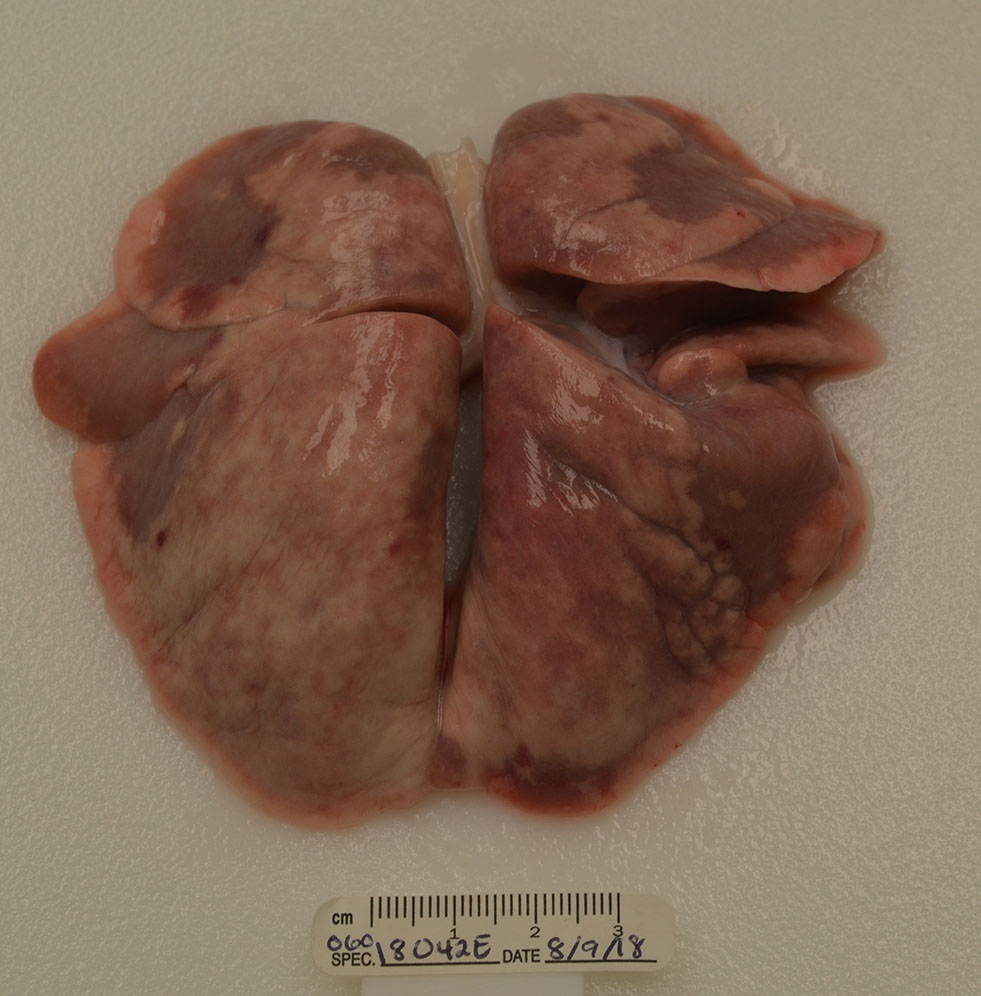
The lungs were more than twice the normal weight with patchy mottled red/brown regions in all lobes (consolidation).
Laboratory Results:
None submitted.
Microscopic Description:
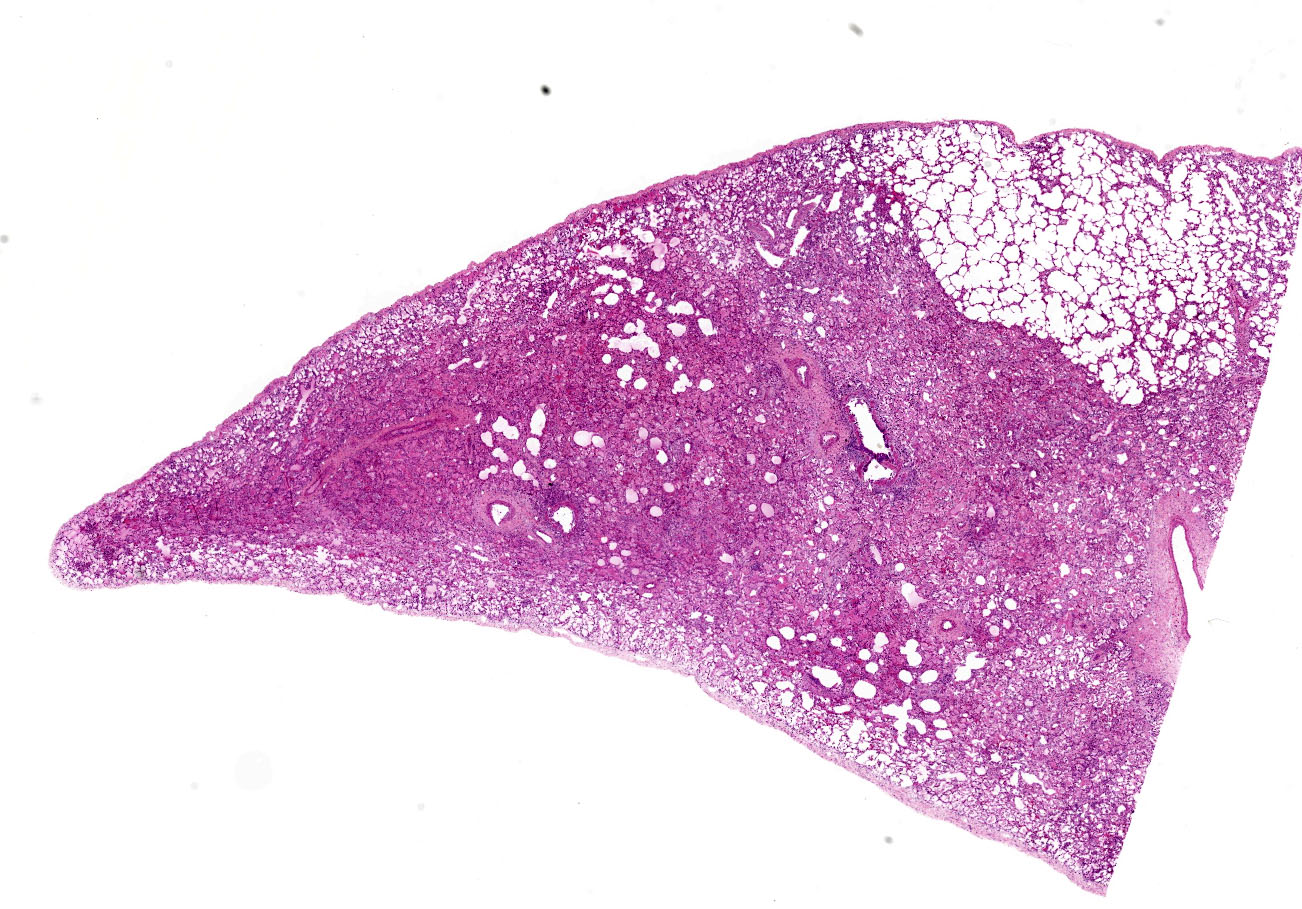
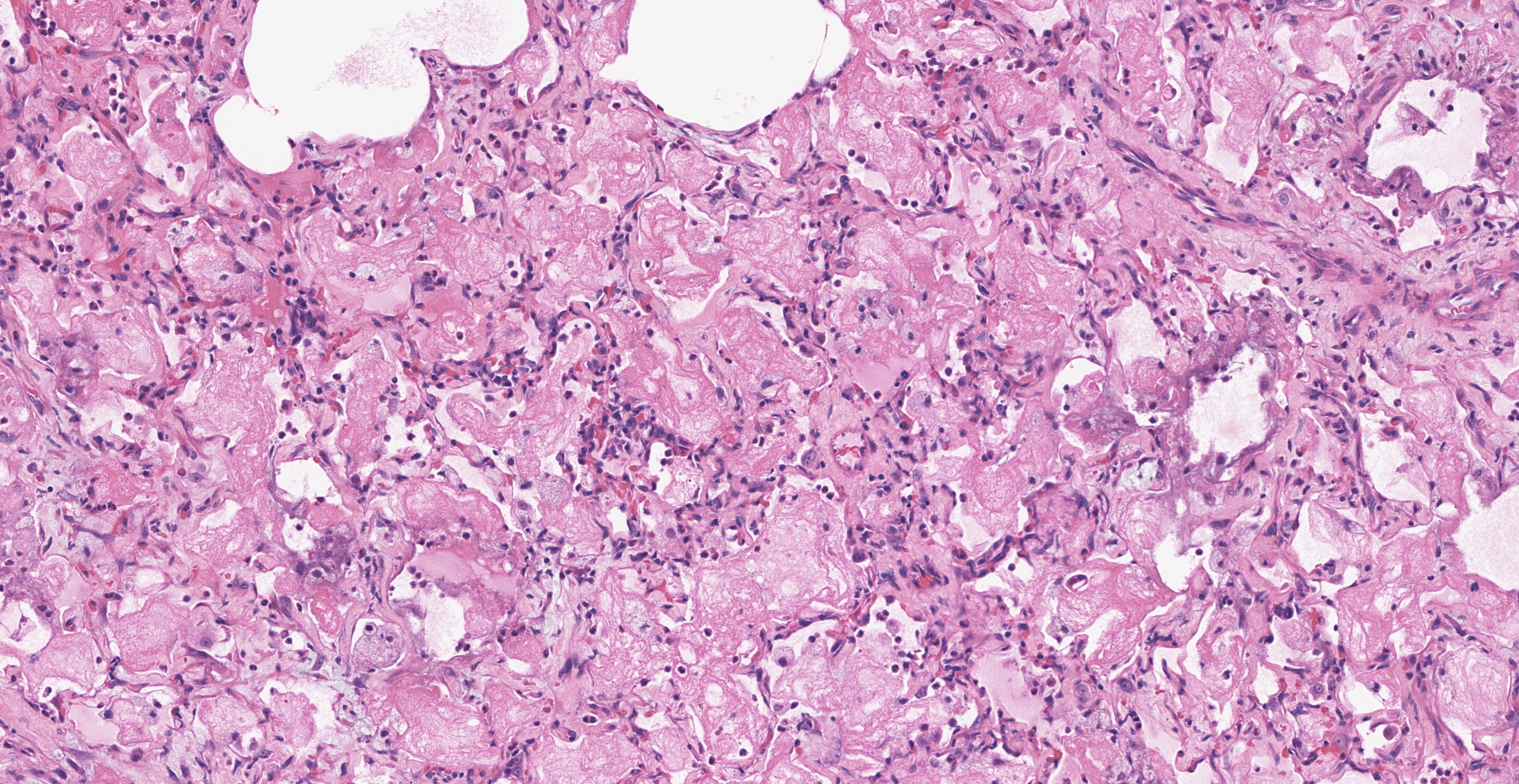
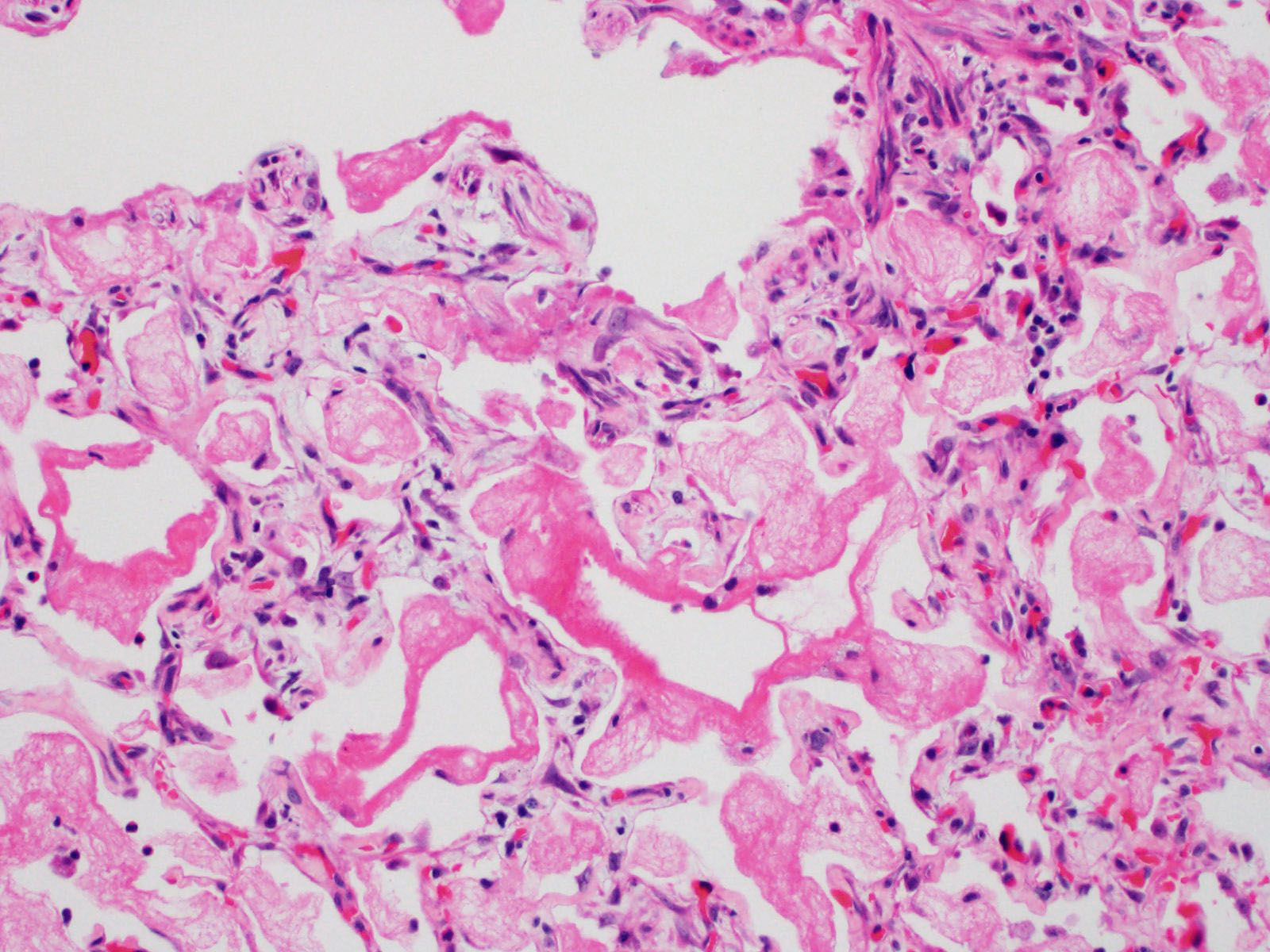
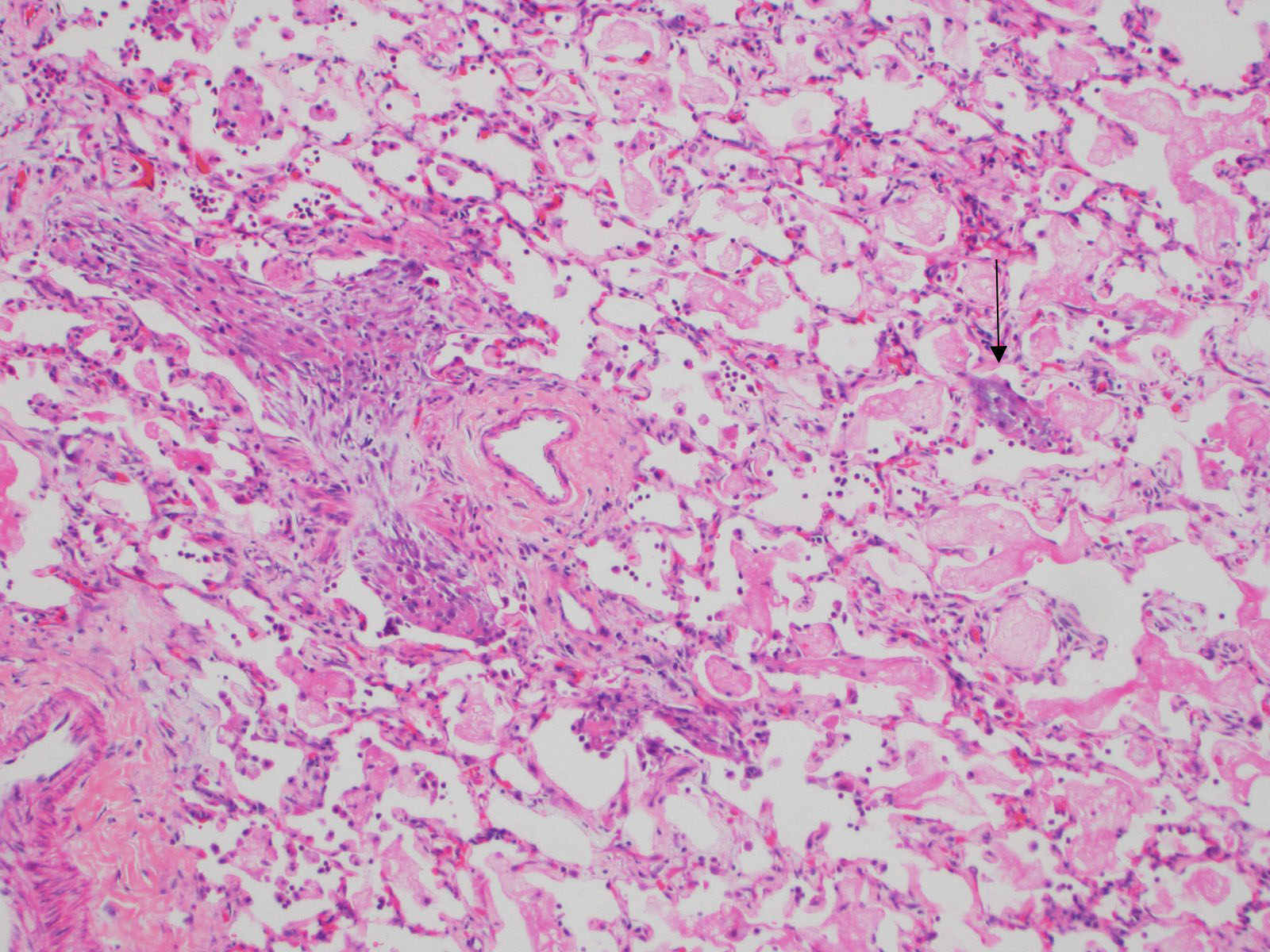
About 80% of alveolar lumina contain eosinophilic homogeneous material (edema), forming hyaline membranes in some, and foamy macrophages and eosinophils. Alveolar spaces are intermittently expanded 2-8 times normal by clear space (emphysema) and adjacent alveoli are collapsed (atelectasis). Alveolar septa are thickened 4-5 times normal by fibrillar eosinophilic material (collagen), eosinophils, plasma cells, and macrophages. Type II pneumocytes are prominent, and many are binucleated with karyomegaly and loss of polarity.
Contributor's Morphologic Diagnoses: Lung: Pneumonitis, diffuse, chronic, severe with alveolar histiocytosis, type II pneumocyte hyperplasia and dysplasia, and interstitial fibrosis.
Contributor's Comment:
Findings in this case are consistent with exposure to ionizing radiation. Radiation-induced lung injury has been divided into two syndromes.3,6 The first is radiation-induced pneumonitis which occurs within months of exposure and is further subdivided into phases. The latent phase lacks histologic damage and is clinically silent. Ultrastructural studies, however, have revealed increased capillary permeability, degenerate mitochondria, and abnormal lamellar bodies in type II pneumocytes. The exudative phase is characterized by excessive leakage of fibrin into alveolar spaces which contributes to the formation of hyaline membranes. During this acute pneumonitis, alveoli are hypercellular primarily due to foamy macrophages. The second syndrome is the late stage of radiation-induced lung injury and is characterized by pulmonary fibrosis. This phase is associated with abundant collagen fibers in the interstitium. Radiation-induced fibrosis then results in progressive dyspnea due to decreased lung compliance and impaired gas exchange.
The extent to which cells are damaged by radiation is based on both physical and biological factors.3 Physical factors include the dose of radiation, usually expressed in Gray, and the period of time over which the radiation is delivered (single, fractionated, or protracted). Biological factors include features of the cell type exposed and how often it divides. Rapidly proliferating cells are more sensitive to injury with irradiation, and differentiated cells are less sensitive. For example, proliferating hematopoietic stem cells in the bone marrow are relatively sensitive while differentiated nervous system cells are resistant. For single dose whole-chest exposures in humans, the dose-response curve is steep, with a 50% incidence of pneumonitis at around 10 Gy.10 The dose-response relationship is similar for macaques.2,8 Concurrent cardiac effects may contribute to pulmonary edema or pleural effusion.
As ionizing radiation passes through tissue, it interacts with intracellular water to generate highly reactive free radical species. Anti-oxidants or detoxifying enzymes are then upregulated to enhance cellular defense mechanisms in response. The overexpression of manganese superoxide dismutase during the late fibrotic response in a mouse model of radiation-induced pneumonitis is one example.5 If injury persists irradiation disrupts the cellular redox balance because of the production of abundant reactive oxygen species. This leads to oxidative damage to DNA, protein, and lipid peroxidation which incites an inflammatory response.9 Despite extensive research, target cells or clear mechanisms underlying radiation-induced lung injury are not fully understood. Among the inflammatory cells, macrophage polarization is considered as an important axis for the control of fibrosis. Radiation-induced injury activates M1 macrophages leading to the initial stage of radiation-induced pneumonitis, and the M2 phenotype has been linked with radiation-induced fibrosis.4 Among pro-fibrotic cytokines, TGF beta plays a major role in fibrogenesis, particularly in the transformation of fibroblasts to myofibroblasts, which is an important stimulus for the production of extracellular matrix.8
Contributing Institution:
http://www.wakehealth.edu/Comparative-Medicine/
JPC
Diagnosis:
Lung: Pneumonia, interstitial, fibrino-necrotic and eosinophilic, diffuse, severe, with hyaline membranes, interstitial fibrosis, and type 2 pneumocyte hyperplasia.
JPC Comment:
Radiotherapy (RT) is a common and effective component of multiple oncologic treatment protocols and has been associated with decreases in local recurrence, distant metastases, and mortality of certain cancers. However, radiation induced lung injury is an inherent risk of RT, particularly in breast and lung cancer patients as lung tissue will invariably be exposed to the radiation portal.7 Approximately 43% of lung cancer patients receiving a total dose of 27.5Gy or more will develop radiation induced lung injury1 and up to 16% of breast cancer patients are affected by radiation pneumonitis and subsequent fibrosis.7 These patients are affected by the two consecutive syndromes described by the contributor, with radiation pneumonitis occurring <6 months following RT followed by radiation induced pulmonary fibrosis.7
As noted by the contributor, the development of radiation induced lung injury is influenced by factors such as the radiation dose as well as the timespan of exposure. Since individual
cases vary, oncologists must carefully select optimal protocols based on individual patient factors. For example, common breast cancer protocols utilize either hypofractionated (42.56Gy in 16 fractions) or conventionally fractionated (50Gy in 25 fractions) protocols over several weeks. Corticosteroids are commonly used a primary treatment of radiation induced pneumonitis, followed by a tapering schedule.7
Clinically, radiation pneumonitis in human patients is a diagnosis of exclusion after differentials such as disease progression, tuberculosis, and asthma are ruled out, followed by chest radiographs, computed tomography scans, and/or pulmonary function tests. However, antemortem diagnosis of radiation pneumonitis is difficult as there are no definitive radiological or laboratory tests.7
Radiation induced lung injury investigations have commonly utilized murine models, particularly the C57BL/6J strain. Similar to NHPs and humans, the threshold single dose required to elicit pathogenesis also appears to be 10Gy. C57BL/6J mice typically develop radiation pneumonitis 8-16wks following exposure, followed by pulmonary fibrosis starting at 24 weeks. Interestingly, C3H and CBA strains develop radiation pneumonitis earlier and at lower doses than the C57BL/6J, making these strains good models for studies evaluating early radiation induced pneumonitis; however, these strains do not typically develop pulmonary fibrosis and are therefore poor models for the evaluation the full progression of radiation induced lung injury.1
Although the pathogenesis in mice appears to be similar to humans and NHPs, their small size historically required irradiation of the entire thorax, which does not typically correlate with the clinical application of targeted small field RT in human patients. This limitation necessitated the use of larger animal models such as pigs and NHPs to allow for hemi-thorax or smaller targeted fields. However, the recent advent of small animal irradiators has provided researchers with the ability to replicate small field RT in murine models.1
Differentials proposed by the moderator for similar lesions presenting in various species include direct (i.e. inhaled) toxins such as oxygen, ozone, nitrous dioxide, and smoke; indirect toxins such as paraquat, 4-ipomeanol, purple mint, stinkwood, rapeseed, and kale; type III hypersensitivity reactions, and potentially infectious etiologies. Conference attendees also noted increased eosinophils in the examined section, for which the underlying cause is unclear.
References:
- Beach TA, Groves AM, Williams JP, Finkelstein JN. Modeling radiation-induced lung injury: lessons learned from whole thorax irradiation. Int J Radiat Biol. 2020;96(1):129-144.
- Cline JM, Dugan G, Bourland JD, et al. Post-Irradiation Treatment with a Superoxide Dismutase Mimic, MnTnHex-2-PyP5+, Mitigates Radiation Injury in the Lungs of Non-Human Primates after Whole-Thorax Exposure to Ionizing Radiation. Antioxidants (Basel, Switzerland). 2018;7(3):40.
- Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect. 1986;70:261-291.
- Duru N, Wolfson B, Zhou Q. Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World J Biol Chem. 2016;7(4):231-239.
- Epperly M, Bray J, Kraeger S, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5(2):196-208.
- Lombardini, Eric D; Pacheco-Thompson, Michelle E; Melanson MA. Radiation and Other Physical Agents. In: Haschek and Rousseaux's Handbook of Toxicologic Pathology. San Diego, United States: Elsevier Science & Technology; 2013.
- McKenzie E, Razvi Y, Bosnic S, et al. Case series of radiation pneumonitis in breast cancer [published online ahead of print, 2021 Dec 9]. J Med Imaging Radiat Sci. 2021;S1939-8654(21)00296-4.
- Parker GA, Li N, Takayama K, Farese AM, MacVittie TJ. Lung and Heart Injury in a Nonhuman Primate Model of Partial-body Irradiation with Minimal Bone Marrow Sparing: Histopathological Evidence of Lung and Heart Injury. Health Phys. 2019;116(3):383-400.
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21(2):260-292. doi:10.1089/ars.2013.5489
Van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJ. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. Int J Radiat Oncol Biol Phys. 1981;7(4):461-467.