Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 6
October 3, 2018
CASE II: V15-09895-X (JPC 4068932-00).
Signalment: 1 day old, male, Maine Anjou mixed breed, Bos taurus taurus, cattle
History: The owner stated that a large percentage of the spring calf crop was born three to four weeks prematurely. Approximately one half of the calves that lived were lethargic at birth and die. A few of the cows had lost weight and thin despite of eating well.
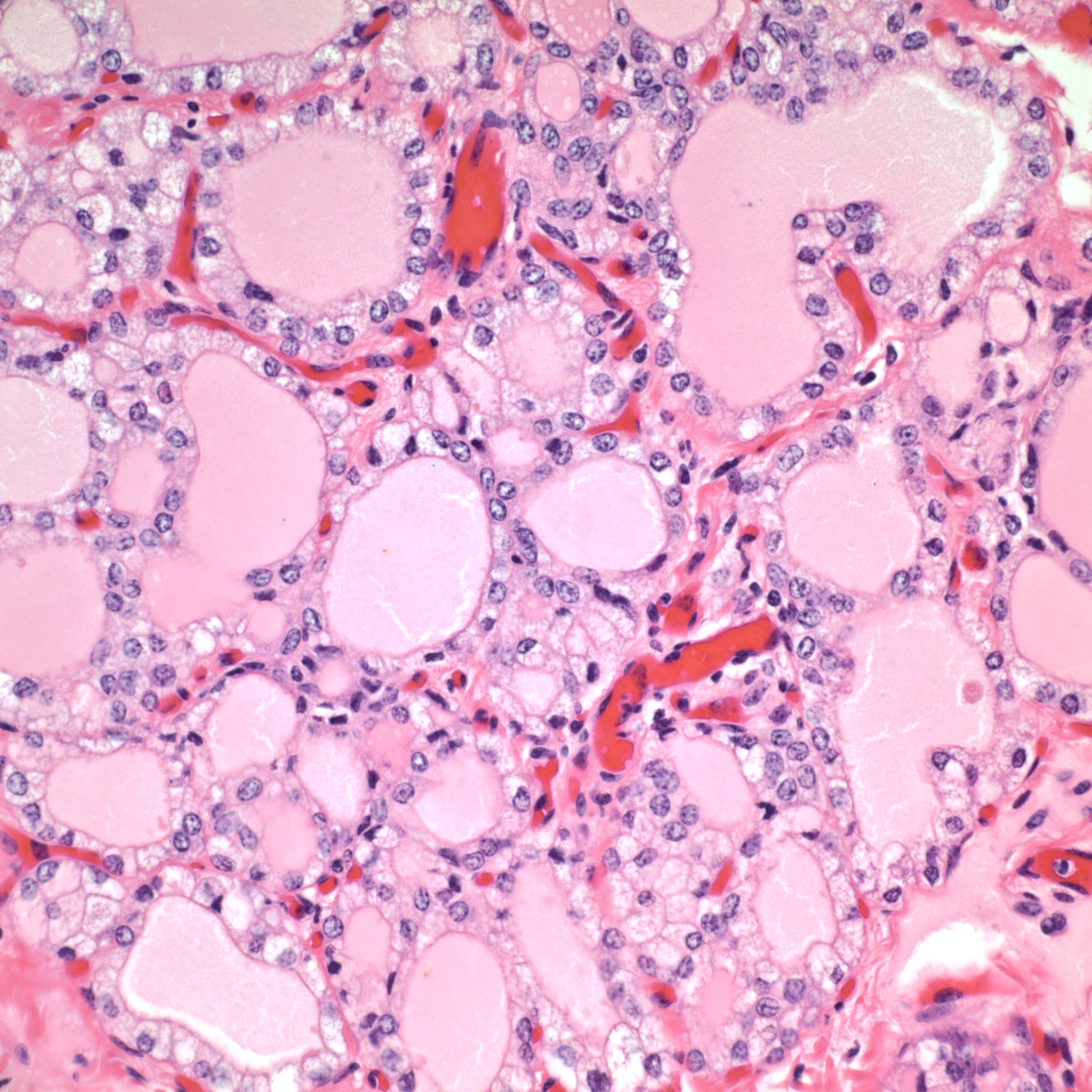
Gross Pathology: The only significant gross lesion was the thyroid gland was equivocally enlarged.
Laboratory results: A plant submitted was identified as Astragulus species by faculty in New Mexico State University Range Sciences.
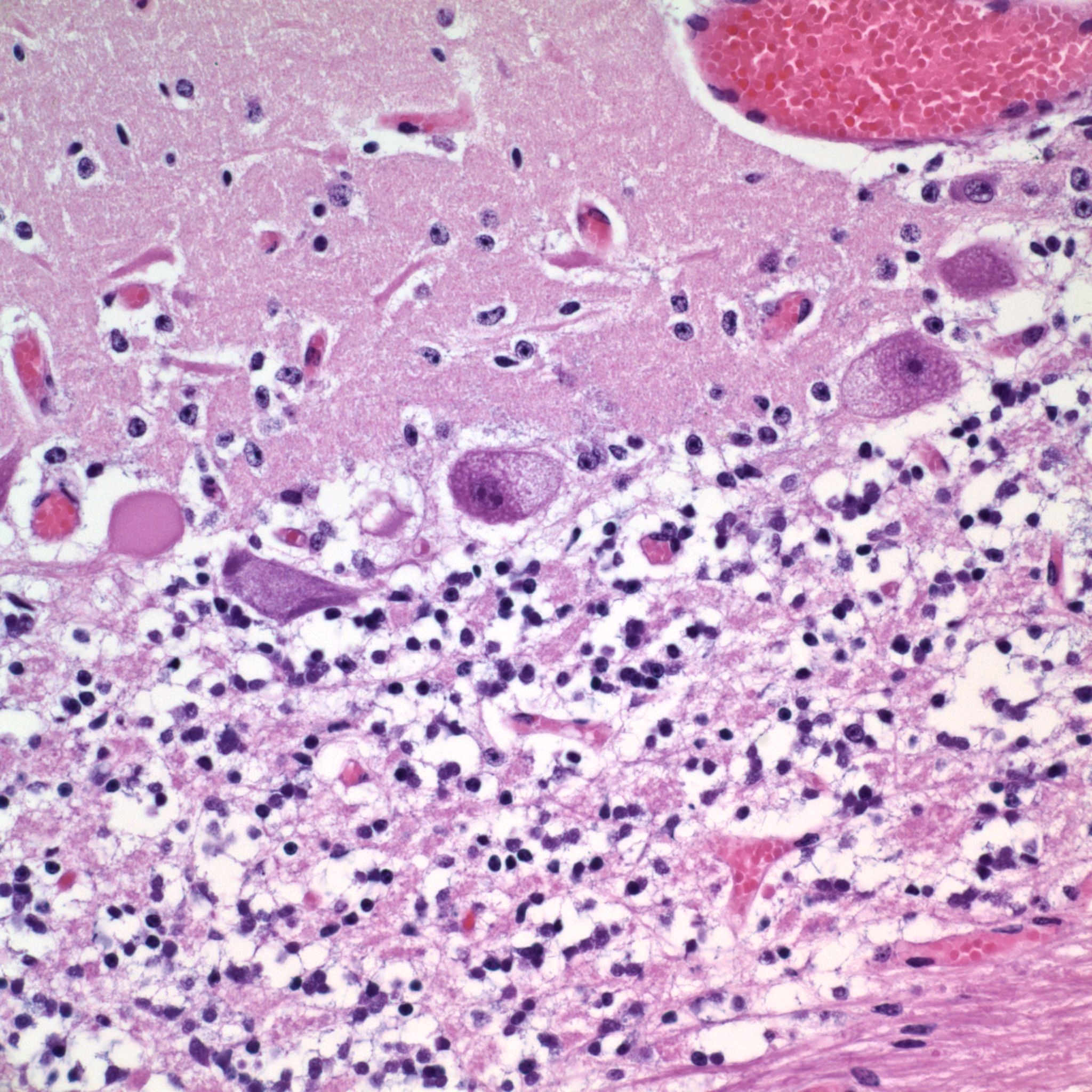
Microscopic Description: The Purkinje cells of the cerebellum are swollen by clear cytoplasmic vacuoles that are most prevalent at axon hillocks (Figure 2). There are rare necrotic Purkinje cells. There are rare affected neurons in the granule cell layer.
Contributor’s Morphologic Diagnoses: Diffuse, severe loss of Purkinje cells (cerebellar abiotrophy)
Contributor’s Comment: Locoism in livestock is caused by the chronic ingestion of legumes of the genera Astragulus and Oxytropis.7,11 There are over 2000 species of Astragulus and Oxytropis worldwide with 370 known species of these legumes occurring in North America.7 Most livestock poisonings caused by these two genera of plants in the United States occur in the western United States.11 Common names for plants of Astragulus and Oxytropis genera are locoweeds, milk vetches or vetches. Not all species of Astragulus and Oxytropis cause locoism. However, these plant species can cause other toxicities as they can contain toxic nitro compounds and can be selenium accumulators.7,11 Identification of a specific species of locoweed is difficult and is best performed by experienced botanists after the plant has flowered and/or produced seed pods.11
The locoweed population is cyclical with the highest number of locoweeds occurring in wet years such as the fall of 2014 and the spring of 2015 in New Mexico.11 Contrary to prior belief, locoweeds are palatable and some animals will preferentially graze locoweeds in the spring over dormant warm season grasses due to the nutritional value of locoweeds, which in some species is comparable to alfalfa.7,11 Animals grazing locoweeds can become habituated to locoweeds, but do not become addicted.7,11 Toxicity occurs when the level of the locoweed toxin reaches the toxic threshold.
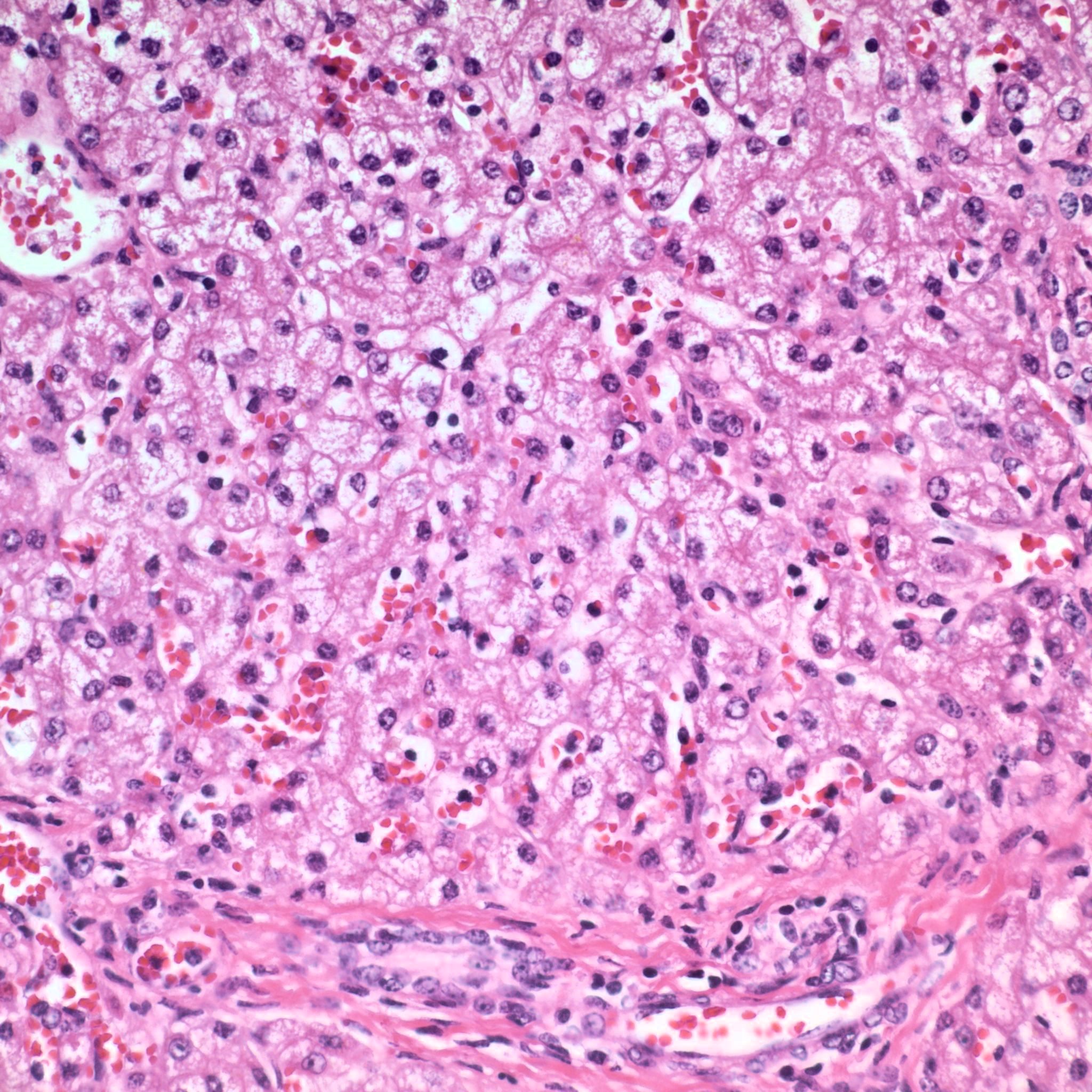
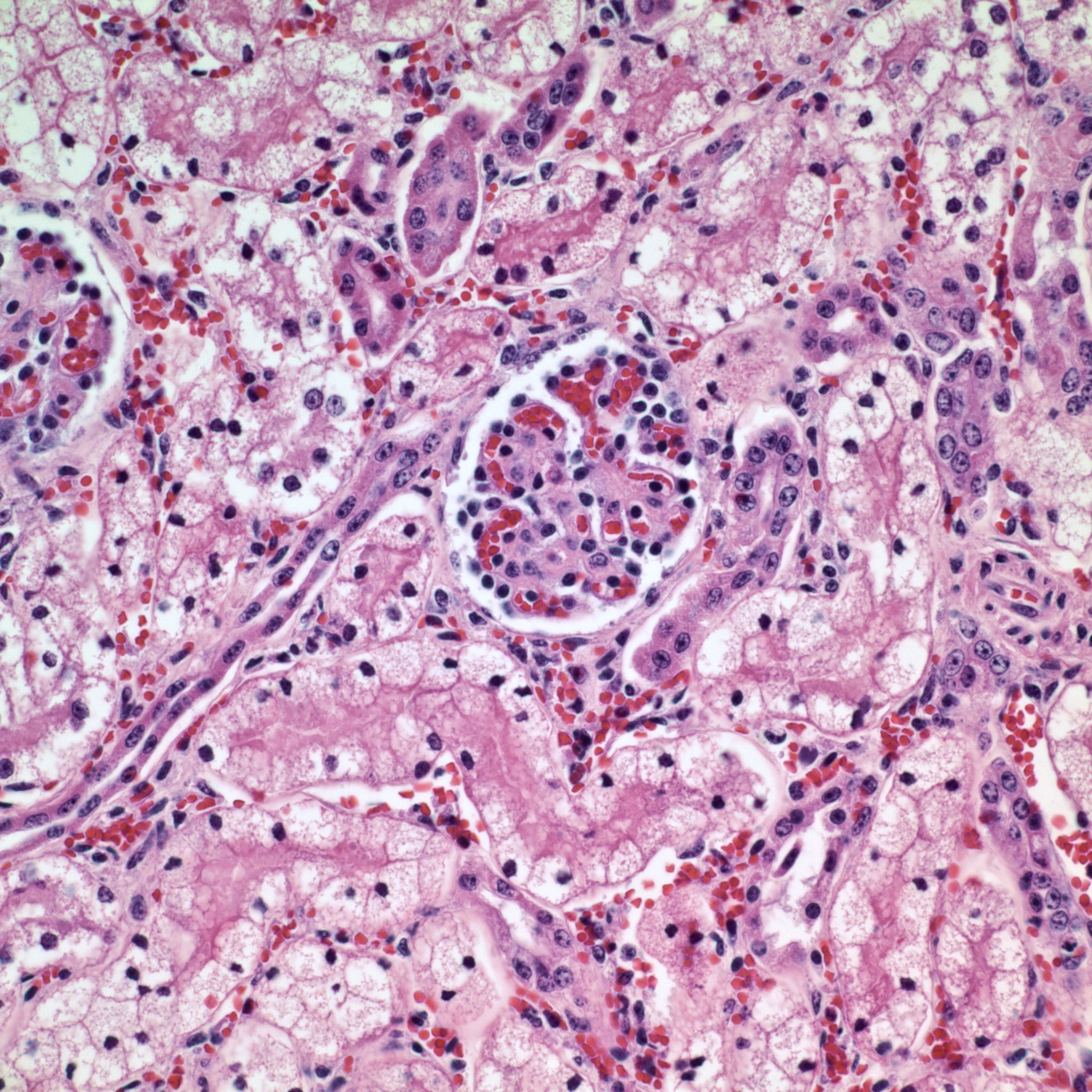
The toxic principle of locoweeds is the indolizidine alkaloid swainsonine.7,11 Swainsonine can be found in all parts of the plant with higher concentrations in the above ground portion of the plants particularly the flowers and seeds.5,7 Production of swainsonine by the plant is dependent on infection of the plant by commensal endophyte fungi of the genera Undifilum.3-6 Swainsonine inhibits α-D-mannosidase and Golgi mannosidase II causing cells to accumulate oligosaccharides.7,11 The result is a systemic lysosomal storage disease with cytoplasmic accumulations of oligosaccharides manifesting microscopically as clear cytoplasmic vacuoles in multiple organs including but not limited to the neurons of the brain mainly in the axon hillock, liver, kidney and thyroid gland.7,8,11 In fetuses, the accumulation of oligosaccharides can also occur in trophoblasts of the placenta.8 If the cellular damage is not severe, then preventing livestock from ingesting locoweeds can result in recovery of the animal with cellular changes remaining the longest in the liver and neurons.7 The cytoplasmic vacuoles can persist for one year in the Purkinje cells of the cerebellum.7 That being said, it is recommended to not ever return horses with locoism to work such as riding due to the potential dangers associated with residual neurological effects.7
The most widely known manifestation of locoweed toxicity in livestock is neurological disease (locoism). However, locoism in livestock can manifest as chronic weight loss/ill thrift, reproductive losses and worsening the cardiac disease associated with high-mountain disease in cattle.7,11 The reproductive losses can be devastating to a producer with losses of close to fifty percent of offspring occurring in some cases. Chronic ingestion of locoweeds has been known to prolong the estrus cycle, result in the failure to conceive and cause early embryonic death.2,7-9 Some pregnant cows with locoism develop hydrops.7 Abortion may occur at all stages of gestation with cytoplasmic vacuoles present in fetal tissues such as in this case.2,7,8 In addition, neonates may be born small and weak. Congenital abnormalities such as limb and head deformities can occur in affected fetuses.2,7,8,9 Testicular atrophy and decreased spermatogenesis can occur in adult males with locoism.7
The moderator pointed out that the cytoplasmic vacuolation is especially prominent in the area of the axon hillock of Purkinje cells in this particular case, a feature often described in this particular entity. Some participant noted the relative paucity of nuclei within the granular layer; the cause of this finding was ascribed by some to the young age of the animal (one day, and with a prominent external granular cell layer, and likely continued maturation of this region), and some preferred to ascribe it to poor tissue preservation.
Contributing Institution:
New Mexico Department of Agriculture Veterinary Diagnostic Services
JPC Diagnosis: Cerebellum, Purkinje and granular cells: Cytoplasmic vacuolation, diffuse, marked.
JPC Comment: The contributor has provided an excellent review of the acquired lysosomal storage disease known as locoism in cattle.
Lysosomal storage diseases are a group of inherited an acquired disorders. Over 50 genetically-determined lysosomal storage diseases have been identified in veterinary medicine.1 The majority of these diseases result from mutations of genes coding specific lysosomal hydrolases which contribute to the activation of lysosomal enzymes. Moreover, infantile and adult types of inherited diseases may result from different mutations of the same gene. Infantile disease results in early death due to a total absence of enzymatic activity, while adult disease may have late presentation and mild-to-moderate manifestations as partial enzyme activity is present. Rare cases of congenital disease may results in the absence of hydroxylase activator proteins, recognition markers (which results in lysosomal enzymes being aberrantly secreted into the extracellular milieu), or protector proteins which results in rapid degradation of hydrolytic enzymes.1 A list of the more common inherited lysosomal storage disease is present Table 1. A more complete review is available in the excellent review by Alroy and Lyons.1
Acquired lysosomal storage disease share a common mechanism in the inhibition of alpha-manosidase II by ingestion of a number of plants from the genera Astragalus, Swainsona, Oxytropis, and Ipomoae as well as a number of amphophilic cationic drugs, including amiodarone and chloroquine. 1
As a general rule, the accumulation of undigested substrate in cells occurs when residual activity of deficient enzymes is less than 10 to 15% of normal levels. There is wide variation in the cell types and organs affected by different types of lysosomal storage diseases; neuronal vacuolation, (as seen in this case), is seen in approximately two thirds of all lysosomal storage diseases.1
Histochemical stains have traditionally been involved in the differentiation of many types of lysosomal storage diseases. Periodic acid-Schiff stains (with and without diastase digestion), Alcian blue, and colloidal iron (with and without hyaluronidase treatment) are useful for identifying oligosaccharide and glycolipids. Sudan Black, oil red O, and Luxol fast blue stains are helpful in the characterization of abnormally stored lipids. Normal ceroid lipofuscinosis is diagnosed by autofluorescence and rarely requires ultrastructure. Definitive identification of enzyme deficiencies is most commonly performed on white blood cells or cultured fibroblasts by measuring activity of specific enzymes.1
The moderator noted that the axon hillock was part of the neuron that was most obviously vacuolated and this was very apparent in this particular specimen. The participants also discussed the possibility of decreased numbers of nuclei within the granular cell layer in this individual and its potential causes, to include the possibility of incomplete maturation in a 1 day old calf (with a very visible external granular cell layer in this case) as well as the possibility of autolysis in the particular specimen.
Table 1: Select inherited lysosomal storage diseases8
|
Condition |
Enzyme Defect |
Storage Material |
Inheritance/species |
|
GM1 gangliosidosis |
β-galactosidase |
GM1 ganglioside in lysosomes of neurons, glial cells, macrophages |
- autosomal recessive |
|
GM2 gangliosidosis (Tay-Sachs and Sandhoff diseases) |
-hexosaminidase (αβ- or ββ-dimer) |
GM2 ganglioside in lysosomes of neurons, glial cells, macrophages |
- autosomal recessive |
|
Sphingomyelinosis (Niemann-Pick disease) |
sphingomyelinase |
sphingomyelin in lysosomes of neurons and macrophages |
- autosomal recessive in cat and dog |
|
Globoid cell leukodystrophy (glactocerebrosidosis) |
galactocerebrosidase |
galactocerebrosides in oligodendrocytes/Schwann cells, globoid cell macrophages (NOT in neurons) |
- autosomal recessive |
|
Glucocerebrosidosis (Gaucher disease) |
glucocerebrosidase |
glucocerebroside in lysosomes of hepatic/lymph node sinusoidal macrophages, some neurons (NOT in Purkinje cells or the spinal cord) |
- Sydney Silky Terriers |
|
α-Mannosidosis |
α-mannosidase |
mannose/N-acetylglucosamine oligosaccharide in lysosomes of neurons, macrophages, secretory epithelial cells |
- Angus cattle |
|
β-Mannosidosis |
β-mannosidase |
oligosaccharides in lysosomes of neurons, macrophages, secretory epithelial cells |
- Salers cattle and |
|
α-L-fucosidosis |
α-L-fucosidase |
- fucose containing glycoconjugates in lysosomes of neurons |
- autosomal recessive |
|
MPS I |
α-L-iduronidase |
mucopolysaccharide storage in mesoderm derived cells |
- domestic shorthair cats |
|
MPS III |
N-acetylglucosamine-6-sulfatase |
heparan sulfate in mesoderm-derived cells; neurons contain gangliosides |
- Nubian goats |
|
MPS VI |
arylsulfatase-B |
mucopolysaccharide storage in mesoderm derived cells; neuronal storage does not occur |
- Siamese and domestic shorthair cats |
|
MPS VII |
β-glucuronidase |
widespread neurovisceral storage |
- dogs and cats |
|
Glycogenosis (type II in humans) |
α-1,4-glucosidase |
widespread glycogen storage within lysosomes and intracytoplasmically; including neurons |
- autosomal recessive in shorthorn and Brahman beef cattle |
References:
- Alroy J, Lyons JA. Lysosomal storage diseases. J Inborn Errors of Metabol Screening 2014; pp 1-20.
- Anderson MA. Disorders of Cattle. In: Njaa BL, ed. Kirkbride’s Diagnosis of Abortion and Neonatal Loss in Animals. 4th Ames, IA. Wiley-Blackwell. 2012 13-48
- Baucom DL, Romero M, Belfon R, Creamer R. Two new species of Undilifum, fungal endophytes of Astragulus (locoweeds) in the United States. Botany. 2012; 90(9): 866-875
- Braun K, Romero J, Liddell C, Creamer R. Production of swainsonine by fungal endophytes of locoweed. Mycol Res. 2003; 107(8): 980-988
- Cook D, Gardner DR, Grum D, et al. Swainsonine and endophyte relationships in Astragulus mollissimus and Astragulus lentiginosus. J Agric Food Chem. 2011; 59: 1281-1287
- Creamer R, Baucom D. Fungal endophytes of locoweeds: a commensal relationship? J Plant Physiol Pathol 2013; 1(2): doi:10.4172/2329-955X.1000104
- Knight AP, Walter RG. Locoism. In: A Guide to Plant Poisoning of Animals in North America. 1st Jackson, WY: Teton New Media: 2001; 204-211
- Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Vol 1. St. Louis, MO: Elsevier; 2007:322-332, 381.
- Moeller RJ. Disorders of Sheep and Goats. In: Njaa BL, ed. Kirkbride’s Diagnosis of Abortion and Neonatal Loss in Animals. 4th Ames, IA. Wiley-Blackwell. 2012; 49-87
- Panter KE, Stegelmeier BL. Reproductive toxicoses of food animals. Vet Clin North Am Food Anim Pract. 2000; 16(3): 531-544
- Panter KE, Welch KD, Gardner DR, et al. Poisonous Plants of the United States. In: Gupta RC, ed. Veterinary Toxicology. 2nd Waltham, MA: Academic Press Elsevier: 2012; 1031-1079