Signalment:
9-month-old, intact female Persian cat (
Felis catus)The
cat had a history of intermittent vomiting and failure to gain weight.
Radiographs revealed a mass at the gastric pylorus and an exploratory
laparotomy was performed.
Gross Description:
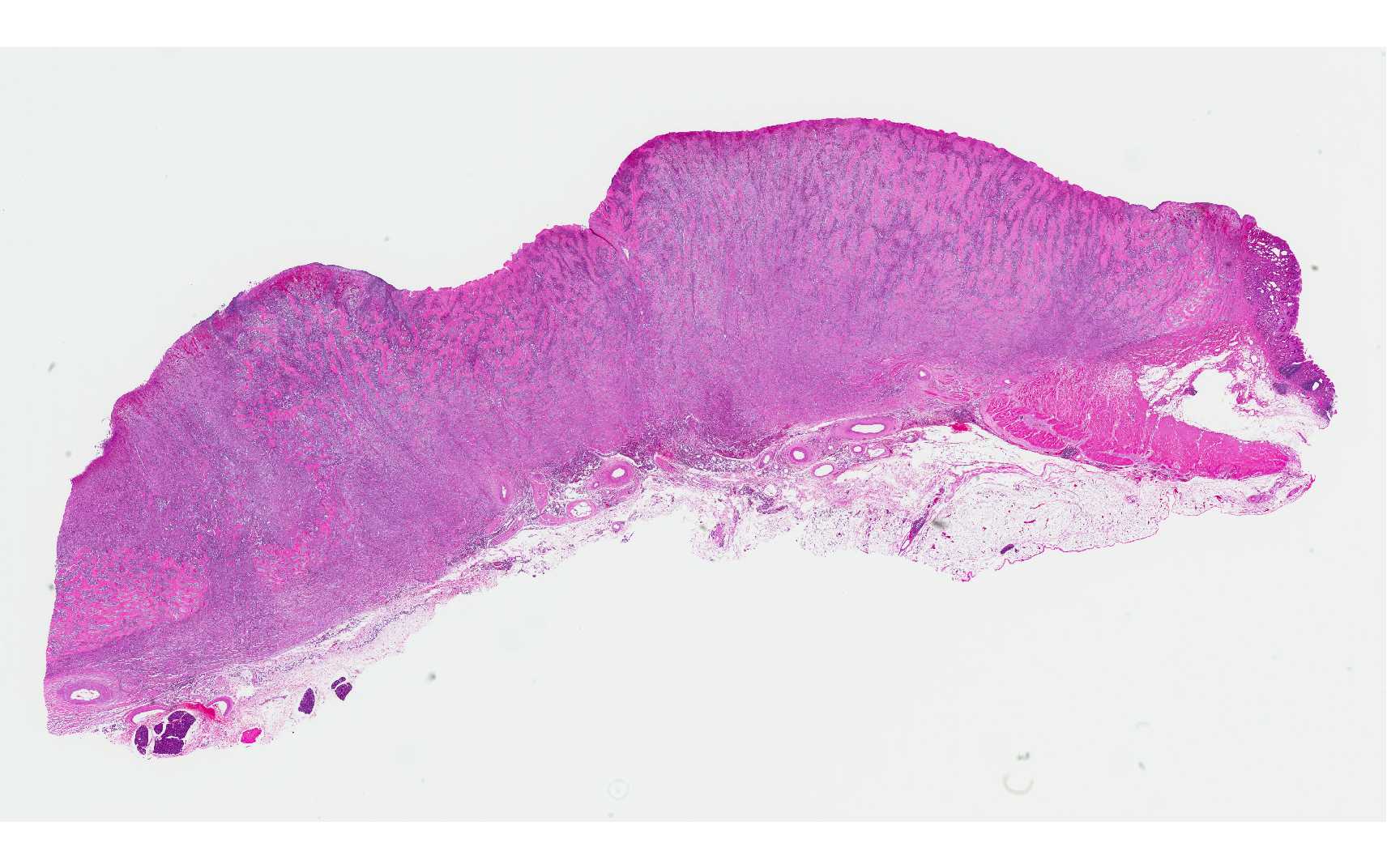
The gastric pylorus region had thickened wall with roughened mucosa surface.
Histopathologic Description:
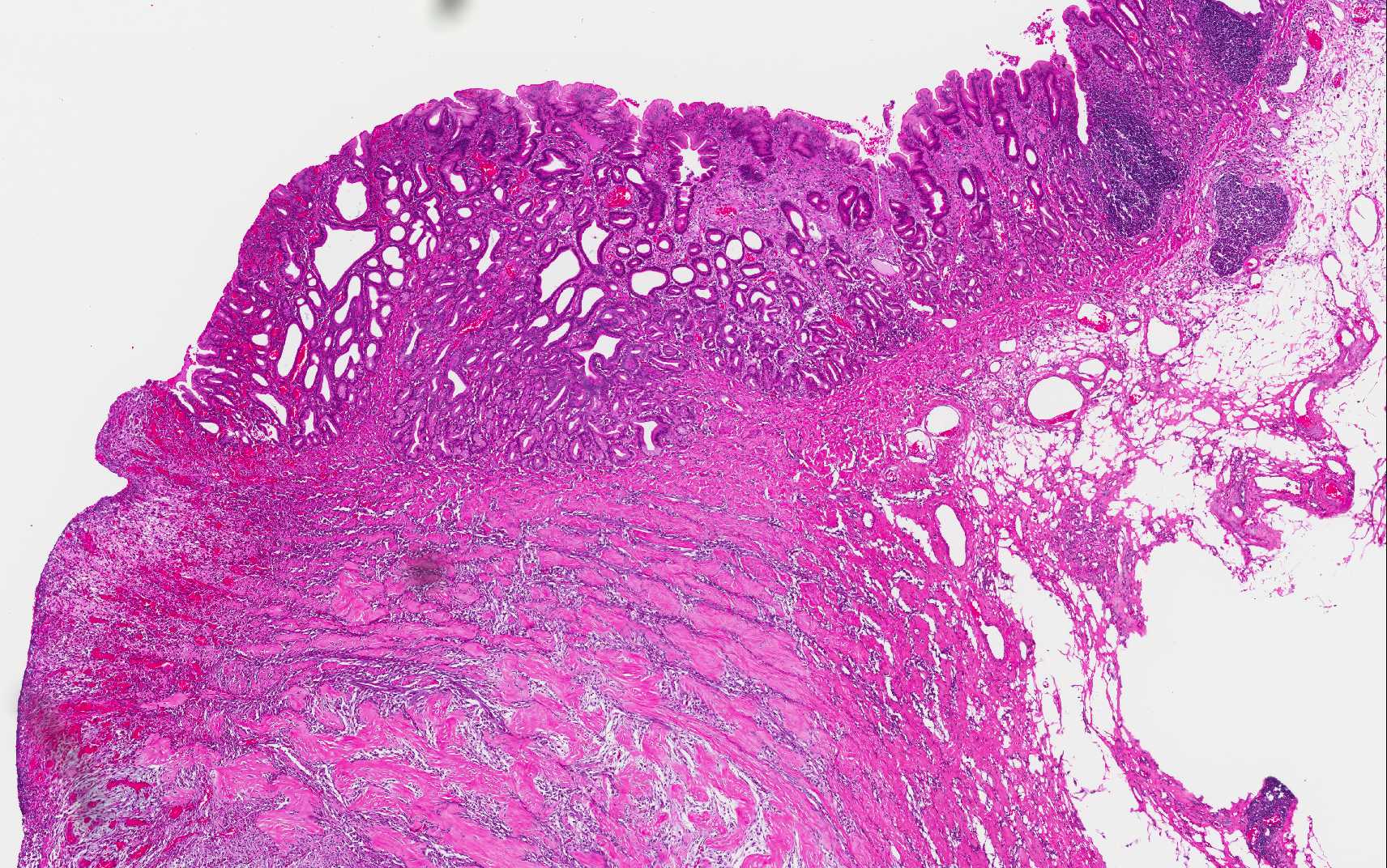
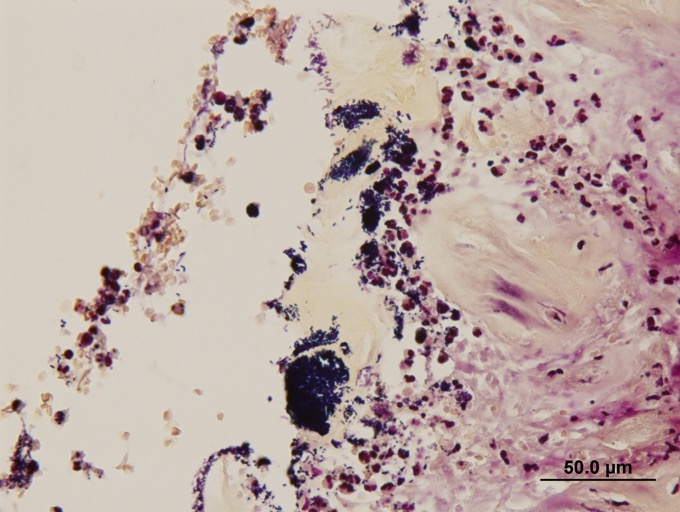
Expanding and effacing the architecture
of the gastric mucosa, submucosa and tunica muscularis at the pyorus is a
transmural proliferation of a fibroproliferative mass with surface necrosis and
ulceration. The lesion affects all layers of the gastric wall, and is comprised
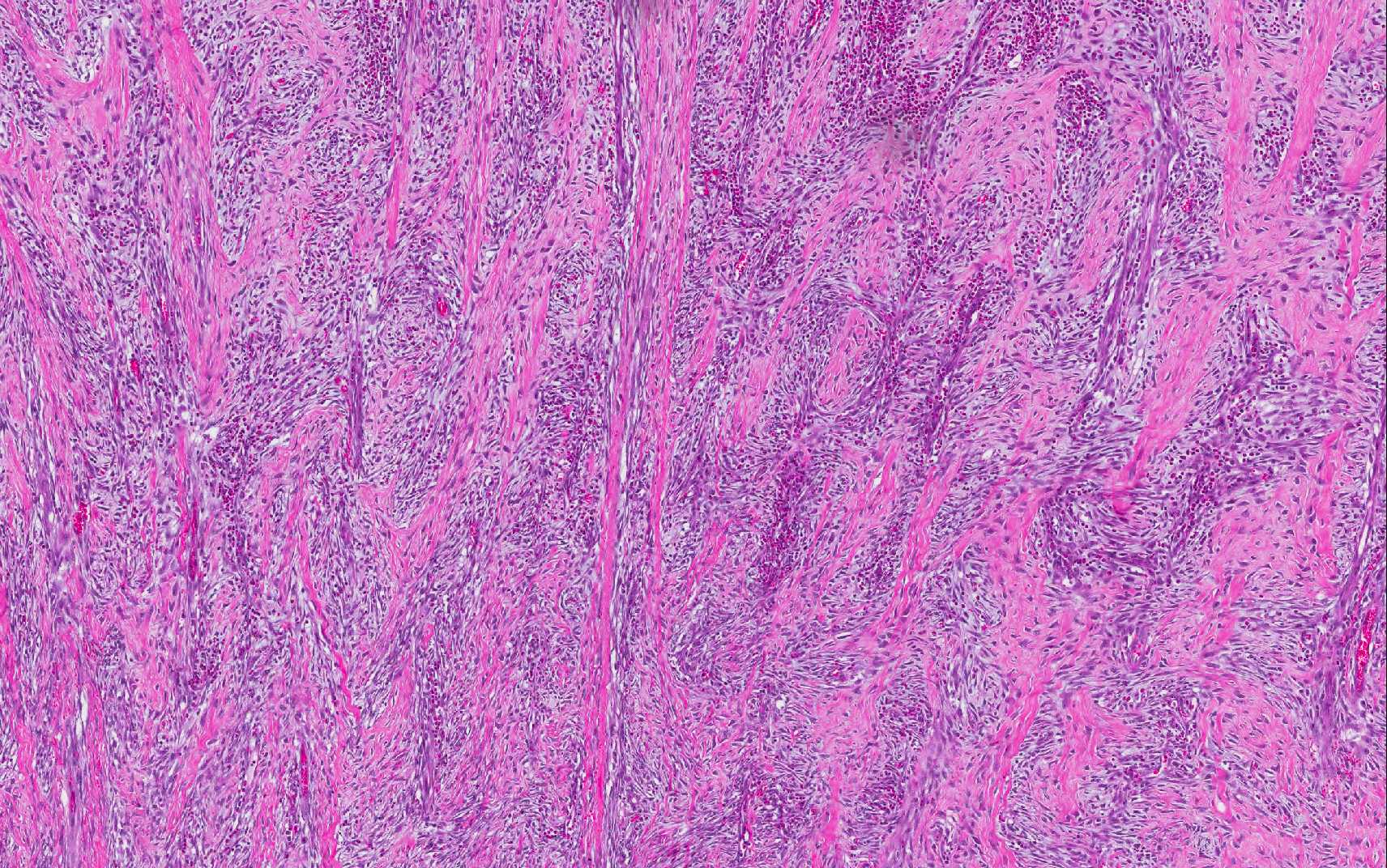
of branching and anastomosing cords and trabeculae of dense collagen separated
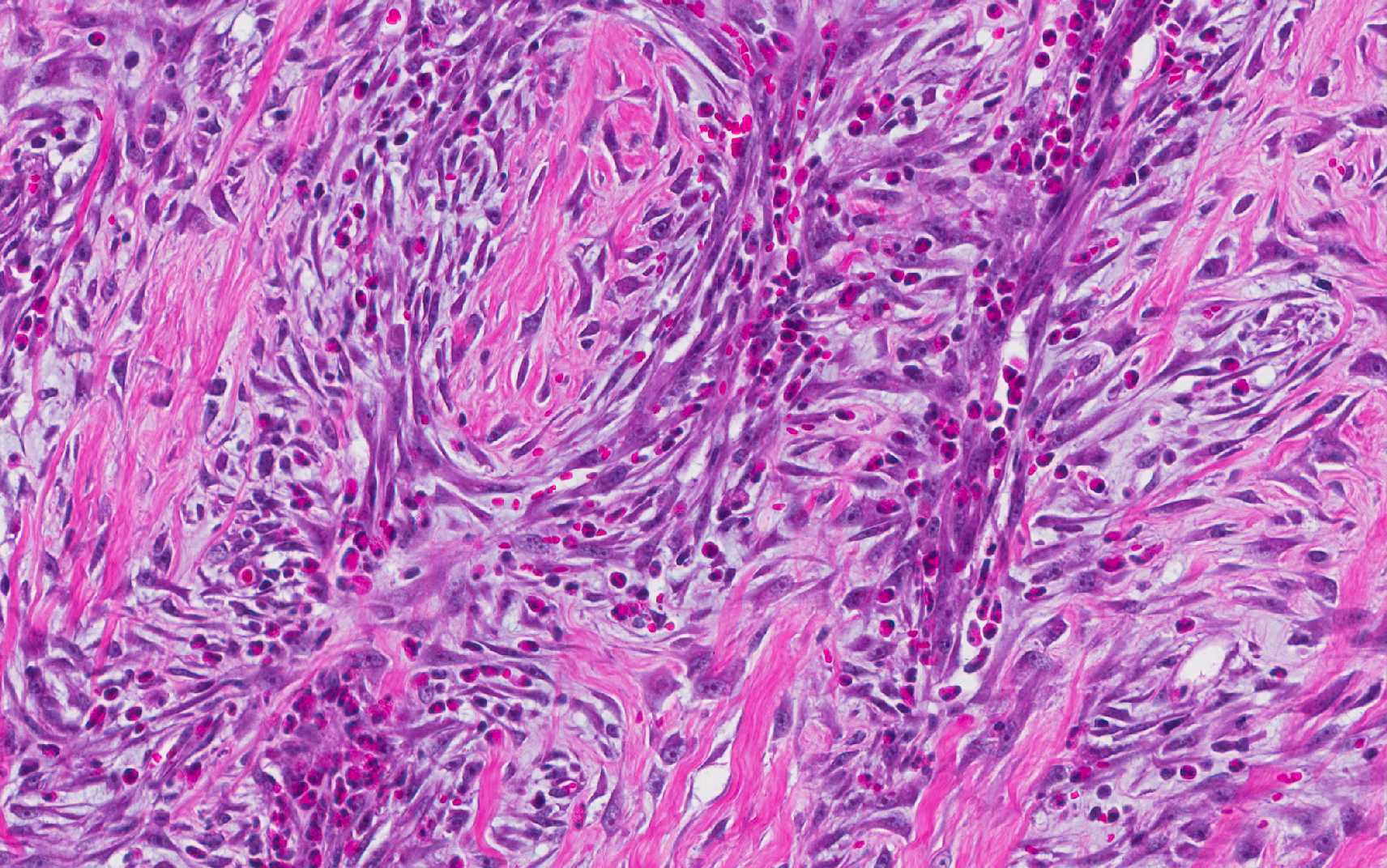
and surrounded by a dense population of fibroblastic cells intermixed with
large numbers of eosinophils, plasma cells, regionally dense neutrophils,
lesser hist-iocytes, mast cells and lymphocytes. Toward the serosal surface,
the fibroblastic proliferation is necrotic and overlain by edematous, loose
fibrovascular proliferation and above described inflammatory cells. There is
obliteration of mucosal epithelium, further covered by necrotic cell debris,
and dense aggregates of neutrophils that are occasionally centered on small
clusters of cocci, as well as hair shafts in cross and tangential section .
Morphologic Diagnosis:
Stomach: 1. Feline
gastrointestinal eosinophilic sclerosing fibroplasia 2. Marked surface
necro-suppurative gastritis, with intralesional hair shafts and bacteria
Lab Results:
None
Condition:
Gastric eosinophilic sclerosing fibroplasia
Contributor Comment:
Feline gastro-intestinal eosinophilic sclerosing fibroplasia is a recently described disease in cats characterized by a nodular, non-neoplastic, proliferative, fibrosing and eosinophilic lesion that effaces the stomach wall of cats.
2 The disease tends to affect middle-aged cats and typical clinical signs include decreased appetite, weight loss, vomiting, and diarrhea.
3
Reported breeds include Ragdoll, domestic shorthair, domestic longhair, Siamese, Maine Coon, Himalayan, Persian and Scottish fold.2,3,4 The disease is most often seen at the pyloric sphincter, ileocecocolic junction or colon, often involving the regional lymph node, and can be associated with peripheral eosinophilia.2 The et-iopathogenesis of this condition is not clearly defined, with migrating foreign body, genetic predisposition and eosinophil dysregulation, herpesvirus infection, and food hypersensitivity are all speculated in the pathogenesis.3
The condition has previously been confused with sclerosing mast cell tumor, which is a neoplasm that tends to occur in the small intestine, unassociated with peripheral eo-sinophilia and extensive fibroproliferation, and less commonly forms palpable masses in the stomach.3 While bacteria are seen within lesions of feline gastrointestinal eosinophilic sclerosing fibroplasia in some reports, antimicrobial therapy is not effective.2 Neither feline coronavirus, feline herpes virus nor any individual bacterial agent have been linked as an etiologic agent.3
A single case report indicated an association between phycomycetes and this disease entity in a domestic cat.5 The disease has been reported in United States, Australia, Europe, Japan and New Zealand 3,4 and this represents this as a first case from Singapore. Prognosis of affected cats is typically grave, and there is no conclusive single conclusive treatment regime.3 Cats treated with prednisolone have a significantly longer survival period and a combination treatment approach of surgical resection, prednisolone, antimicrobial therapy and immune modulation may help improve clinical outcome.3
JPC Diagnosis:
Small
intestine: Enteritis, necrotizing, circumferential, diffuse, severe with
numerous mucosa-adherent bacilli.
Conference Comment:
The mass lesion present in feline gastrointestinal eosinophilic
sclerosing fibroplasia has been described as hard, non-painful and easily
palpable. Upon fine needle aspiration or biopsy the lesions are firm,
described as -Ç-ÿgritty, and are heterogeneous when sectioned at necropsy or at
time of surgery.3 Histologically, it is not uncommon to find
bacteria within the lesions, as seen in this case; fungal organisms and
nematode infections have also been associated with these lesions.3,5
Secondary infections are proposed to play a role in perpetuation of the
inflammatory lesion. The broad trabeculae of fibroplasia intermixed with foci
of inflammation is characteristic of the lesion, as conspicuously observed in
this case. The histologic differential diagnosis includes fibrosarcoma and mast
cell tumor; and malignant lymphoma is also a consideration at the macroscopic
level.
Eosinophils are presumed to play a primary role in the
pathogenesis of this fibroplastic lesion. Eosinophils are most commonly called
in from the bloodstream in response to chemoattractants, such as in parasitic
and allergic conditions, and are often seen as a component of subacute and
chronic inflammation. Eosinophils contain several different types of granules,
including large specific granules, small granules, primary granules, and
secondary granules, which elaborate a wide variety of cytokines, chemokines and
degradative enzymes that can perpetuate and enhance the inflammatory response,
stimulate fibrosis, and result in significant host tissue damage, including
cell and extracellular matrix components. Eosinophil chemottractants originate
from a variety of sources such as epithelial cells, parasites, mast cells and
eosinophils themselves and include CCL-5 (RANTES), C5a, CCL-11 (eotaxin), IL-4,
IL-5 and IL-13.1One important mediator is major basic protein, which
is present within large specific granules; the protein is toxic to helminths,
as well as adjacent host cells, and causes histamine release from mast cells as
well as activating neutrophils.
In this lesion, eosinophils compose a major component of the
inflammatory cell population, with mast cells being relatively fewer in number.
Extracellular bacteria can be visualized without the aid of Gram stains.
Multifocal areas of inflammation, composed of neutrophils, macrophages and
multinucleate giant cells, surround free hair shafts.
References:
1. Ackermann MR. Female reproductive system and mammary gland. In: McGavin MD, Zachary JF, eds.
Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:102.
2. Craig LE et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia.
Vet Pathol. 2009 Jan; 46(1):63-70.
3. Linton M et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J
Feline Med Surg. 2015 May; 17(5):392-404.
4. Suzuki M, Onchi M, Ozaki M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia.
J Toxicol Pathol. 2013 Mar; 26(1):51-3.
5. Grau-Roma L, Galindo-Cardiel I, Isidoro-Ayza M, Fernandez M, Maj³ N. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with phycomycetes.
J Comp Pathol. 2014 Nov; 151(4):318-21.