Signalment:
Gross Description:
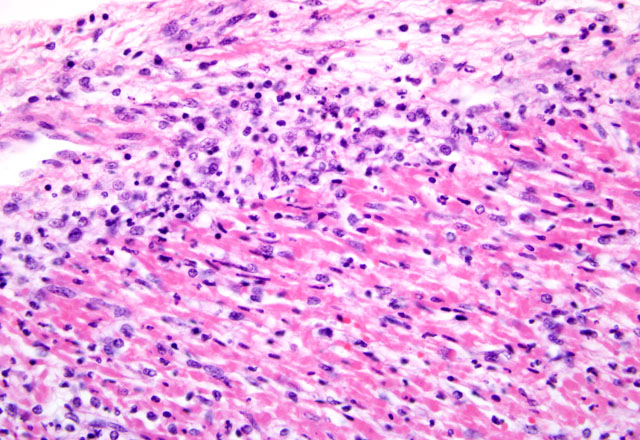
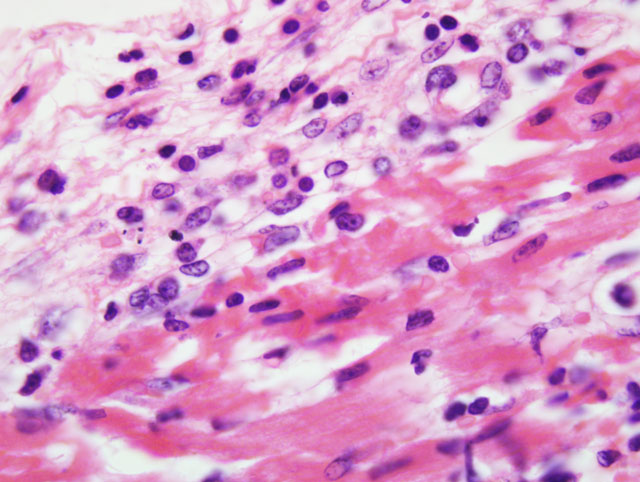
Histopathologic Description:
Morphologic Diagnosis:
Aetiologic diagnosis: Aphthovirus myocarditis
Lab Results:
Heart, nail, tongue and pericardial fluid samples were positive for FMDV, type O, Southeast Asia (SEA) topotype, Mya-98 lineage, by nucleotide sequencing.(10) Results were from the World Foot and Mouth Disease Reference Laboratory at Pirbright.
Condition:
Contributor Comment:
FMDV is a single stranded RNA virus with a protein capsid consisting of four viral proteins enumerated as VP1, VP2, VP3 and VP4.(1) Subtype-specific neutralization epitopes have been identified among the four capsid proteins, primarily within VP1.(6,8) The serotype prevalent in Hong Kong SAR has been and continues to be serotype O.(4) However, the topotype has recently changed from Cathay to the South East Asia topotype with a subsequent increase in clinical signs and mortalities. The Southeast Asia (SEA) topotype, Mya-98 lineage, has been confirmed by the World Foot and Mouth Disease Reference Laboratory at Pirbright. Similar topotypes have been identified in the Republic of Korea and Japan recently. This recent spread of FMD in the Far East indicates the possibility of disease spread both within and outside the region, as happened during the FMD O PanAsia pandemic of 1999-2001.(4,10)
In swine, vesicles often form on the rostrum of the snout and around the nares. Foot lesions, when present, frequently extend along the coronary bands and dewclaws, and in severe cases can cause sloughing of the claw. Tongue lesions tend to coalesce and ooze a serous fluid when ruptured, leaving behind a visible raw and denuded stratum germinativum.(6)
Mortality is not commonly seen in FMD cases unless young pigs are affected. Young pigs up to 14 weeks of age, but particularly those less than 8 weeks of age, may die without developing any clinical signs of FMD due to heart failure, which is characterized by acute myocarditis that at times may be seen macroscopically as whisps of blanched areas on the epicardium that extend into the endocardium, a consequence of the damage caused by viral replication to the developing myocardium.(3,9,11)
JPC Diagnosis:
Conference Comment:
The exact cause of death in myocarditis is unknown. Death could be due to the effects of the offending agent (e.g. viral damage to myocytes), the ensuing inflammatory response, or both. Infiltrating cytotoxic T lymphocytes can produce direct myocyte necrosis. Activated histiocytes and lymphocytes produce a cytokine milieu resulting in myocyte metabolic disturbances, reduced contractility, and dysrhythmia. Death during the acute phase of myocarditis is often attributed to dysrhythmias.(7) Animals surviving the acute phase begin resolution in which macrophages arrive to remove the necrotic debris. Due to the continuous contraction of cardiac myocytes, their regenerative capabilities are limited; therefore, healing most often occurs through fibrosis.(5)
References:
2. Hoffmann B, Beer M, Reid S, et al. A review of RT-PCR technologies used in veterinary virology and disease control: Sensitive and specific diagnosis of five livestock diseases notifiable to the World Organization for Animal Health. Vet Microbiol. 2009;139:1-23.
3. Kitching RP, Alexandersen S. Clinical variation in foot and mouth disease. Rev sci tech Off int Epiz. 2002;21(3): 513-518.
4. Knowles NJ, Samuel AR, Davies PR, Midgley RJ, Valarcher J-F. Pandemic strain of Foot-and-Mouth Disease Virus Serotype O. Emerg Infect Dis. 2005;11(12):1887-1893.
5. Kumar V, Abbas AK, Fausto N, Aster JC. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2009:85-86.
6. Lubroth J. Foot and mouth disease: A review for the practitioner. Vet Clin Food Anim. 2002;18:475-499.
7. Maxie MG, Robinson WF. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 3. Philadelphia, PA: Elsevier Ltd; 2007:41-43.
8. Meyer RF, Knudsen RC. Foot and mouth disease: a review of the virus and the symptoms. J Envir Health. 2001;64(4):21-23.
9. Oeem JK, Yeh MT, McKenna TS, et al. Pathogenic characteristics of the Korean 2002 isolate of Foot and Mouth Disease Virus serotype O in pigs and cattle. J Comp Path. 2008;138:204-214.
10.Paton DJ, King DP, Knowles NJ, Hammond J. Recent spread of foot-and-mouth disease in the Far East. Vet Rec. 2010;166:569-570.
11.Ryan E, Horsington J, Durand S, et al. Foot and mouth disease virus infection in young lambs: pathogenesis and tissue tropism. Vet Microbiol. 2008;127:258-274.
12.Van Vleet JF, Ferrans VJ. Cardiovascular system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:563-564, 591-593.