Signalment:
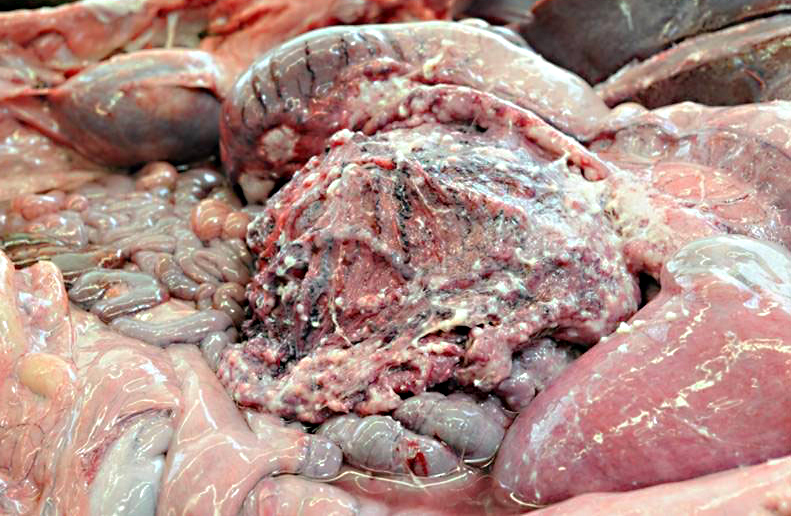
Gross Description:
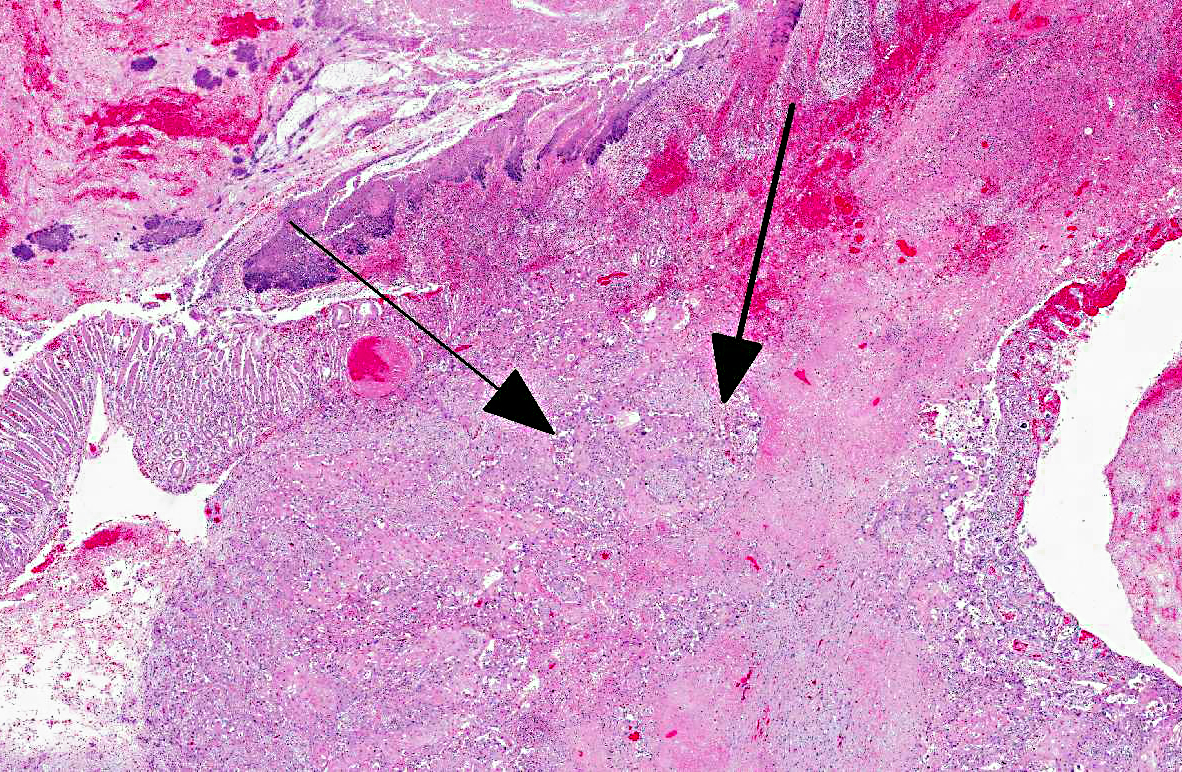
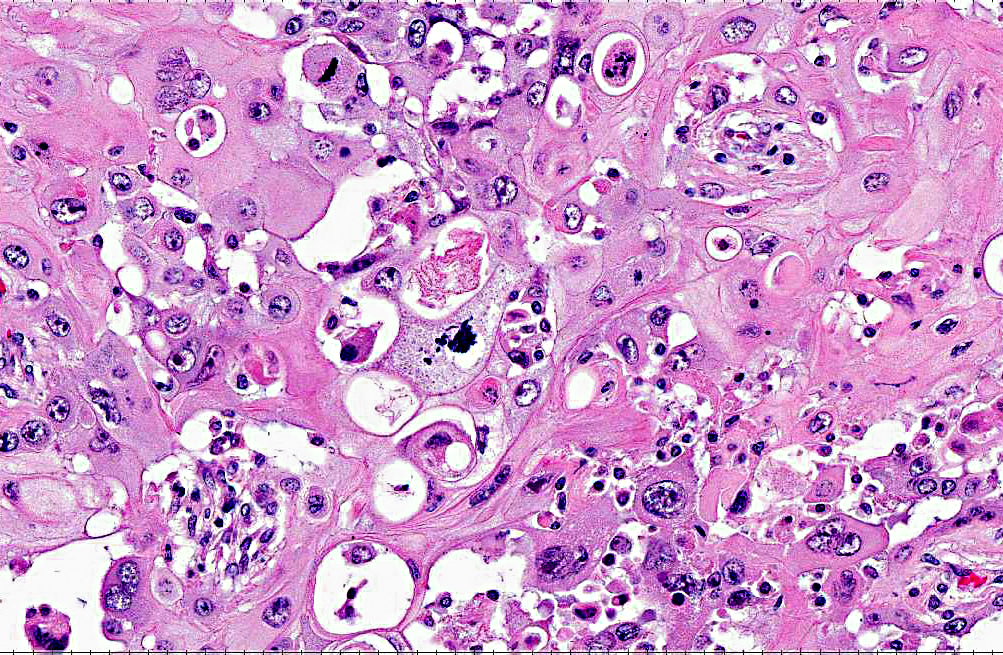
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
There are numerous reports of neoplasia in South American camelids, including lymphoma, urogenital tumors, cutaneous and mucocutaneous neoplasia, oral, intraocular, gastrointestinal, pulmonary, neuroendocrine and brain neoplasia (Table 1).(5)
Gastric squamous cell carcinoma in ruminants is usually quite rare and only few have been reported in llamas and alpacas.(3,5) However, in a 5 year (2001-2006) study at Oregon State University it was reported that cutaneous and mucocutaneous squamous cell carcinoma (SCC) was the most frequent malignant neoplasm identified, although in previous studies lymphoma was most commonly reported.(5)
Histologically, C1 and portions of C2 are composed of non-keratinized stratified squamous epithelium, whereas the rest of C2 and all of C3 have a mucinous glandular epithelium.
In previous cases, neoplastic cells most commonly originated from squamous mucosal epithelium of compartments 1 and 2, but it could arise also from the glandular mucosa of compartment 3.(3) In this case, the neoplasm was most closely related to the stratified squamous epithelium of C2. SCC of glandular mucosa could derive from metaplasia of glandular epithelium, from growth of heterotopic squamous cell rests, or from multipotent cells of the crypt gland base, as suggested in prevous reports in dogs.(3)
Commonly described sites of metastases are the liver, diaphragm, mesentery, and myocardium. The mode of metastatic spread is by implantation and vascular dissemination, as seen in horses with gastric SCC.(3)
Several factors have been associated with the development of gastric papillomas and squamous cell carcinomas in humans and other species including host, dietary, genetic, environmental conditions and infectious agents. In cattle, papillomas of the esophagus and reticulorumen are caused by bovine papillomavirus 4 (BPV-4), whereas fibropapillomas of the esophagus, esophageal groove and rumen are caused by bovine papillomavirus (2). It is very seldom for viral particles to be seen in the tumor. Malignant neoplasia of bovine esophagus and forestomach is extremely rare.(2,3) On the contrary, SCC involving male and female genitalia, ocular and periocular tissue and stomach is most commonly reported in the horse. Equine gastric SCC metastasized most frequently to retropharyngeal lymph nodes.(4)
The frequency of llamas and alpacas as patients in the veterinary hospital is increasing; therefore it is important to improve the knowledge about possible lesions, prevalence and predisposing factors causing diseases in these animals. Table 1. Summary of 40 tumors reported in 20 llamas and 18 alpacas between 2001-2006 at Oregon State University.(5)
| Tumor type | Total no. tumors | Alpacas (no.) | Llamas (no.) | Mean age alpacas (yrs)* | Mean age llamas (yrs) | Location(s) | |
| Fibroma/fibropapilloma | 12 | 8 | 2 | 5.6 | 11.5 | Face; nose; lips; dista limb; gingiva; hard palate | |
| Carcinoma | |||||||
| Cutaneous Ocular Mammary Un-differentiated | 4 2 2 1 | 0 1 0 1 | 4 1 2 0 | -- U -- 12 | 13.5 14 17 -- | Perineum trunk; limb 3rd eyelid Metastatic to local lymph node; disseminated Disseminated |
|
| Adenocarcinoma | |||||||
| Biliary Pancreas Intestine | 2 1 1 | 0 1 0 | 2 0 1 | -- 10 -- | 10.5 -- 8 | Disseminated Disseminated Disseminated |
|
| Lymphoma | 5 | 4 | 1 | 1.5 | 15 | Disseminated | |
| Fibrosarcoma | 4 | 1 | 3 | 6 | 13 | Lips; gingiva; maxilla; cornea | |
| Lipoma | 2 | 0 | 2 | -- | 12 | Mesentery; subcutis | |
| Melanocytoma | 1 | 0 | 1 | -- | 11 | Pectoral skin | |
| Leiomyosarcoma | 1 | 0 | 1 | -- | 12 | Uterus | |
| Interstitial cell tumor | 1 | 1 | 0 | 9 | -- | Ovary | |
| Primitive stromal tumor | 1 | 1 | 0 | U | -- | Testis | |
| * U = unknown | |||||||
JPC Diagnosis:
Conference Comment:
Following is a brief summary of new world camelid gastric anatomy and histology: As the contributor described, new world camelid stomachs are composed of three compartments :C1, C2, and C3, which are sometimes referred to as the proximal compartment (PC), intermediate compartment (IC) and the distal compartment (DC), respectively. C1 is often compared with the rumen, and constitutes the largest chamber (comprising approximately 83% of the gastric volume). C2 is somewhat kidney-shaped and is the smallest of the three compartments. C3 is tubular and elongated and accounts for approximately 11% of the gastric volume. Histologically the majority of C1 is nonglandular, covered by stratified squamous epithelium which is supported by a dense collagenous lamina propria. This tissue is arranged in folds (rather than papillae as in ruminants) which are more prominent when the stomach is contracted. Smaller, more ventral portions of C1 (known as cranial and ventral glandular saccules) are lined by columnar epithelium and deep tubular glands which are supported by a smaller amount of looser connective tissue. C1 communicates with C2 through the ventricular furrow. C2 has thick walls, and histologically is divided into two regions: a dorsal nonglandular and a ventral glandular portion, each of which are similar to those described for C1. Lymphoid tissue may be found in the lamina propria of the glandular portions of C2. The muscularis mucosae is absent in the nonglandular regions of both C1 and C2, and is present in glandular areas, but is incomplete. C2 communicates with C3 via the channel of isthmus, a small tubular continuation of the ventricular furrow that is lined by stratified epithelium. C3 is otherwise covered by a glandular mucosa, with 3 regions differing histologically: the proximal region has abundant lymphoid tissue in the mucosa and submucosa; the central region has simple mucous-secreting tubular glands; and the caudal region has fundic glands in the ventral portion and simple tubular glands in the dorsal (pyloric) region. C3 has a well-developed muscularis mucosae and a thin submucosa. Surrounding all three gastric compartments is a muscularis comprised of inner circular and outer longitudinal layers. (1)
References:
2. Mckenzie EC, Mills JN, Bolton JR. Gastric squamous cell carcinoma in three horses. Aust Vet J. 1997;75(7):480-483.
3. Sartin EA, Waldridge BM, Carter DW, et al. Gastric squamous cell carcinoma in three llamas. J Vet Diagn Invest. 1997;9:103-106.
4. Schuh JCL. Squamous cell carcinoma of the oral, pharyngeal and nasal mucosa in the horse. Vet Pathol. 1986;23:205-207.
5. Valentine BA, Martin JM. Prevalence of neoplasia in llamas and alpacas (Oregon State University, 2001-2006). J Vet Diagn Invest. 2007;19:202-204.