CASE I:
Signalment:
4-month old, male common marmoset (Callithrix jacchus)
History:
This 4-month old, male marmoset presented for being down in his cage and unresponsive. On physical exam, he was noted to be laterally recumbent, hypothermic, and dehydrated. The animal was minimally responsive to repeated supportive care attempts, and humane euthanasia was elected, followed by a necropsy. Prior to presentation, a sibling in this same family was submitted for necropsy, where post-mortem culture and subsequent serotyping identified Salmonella typhimurium.
Gross Pathology:
At necropsy, the stomach was filled with tan to white feed material. The small intestines were variably distended with gas and contained scant to moderate mucoid ingesta. Additionally, a focal area of necrosis (~1 cm in diameter) was observed on the left lateral liver lobe.
Laboratory Results:
Blood smear prior to euthanasia demonstrated few neutrophils, mostly bands, with evidence of toxic change.
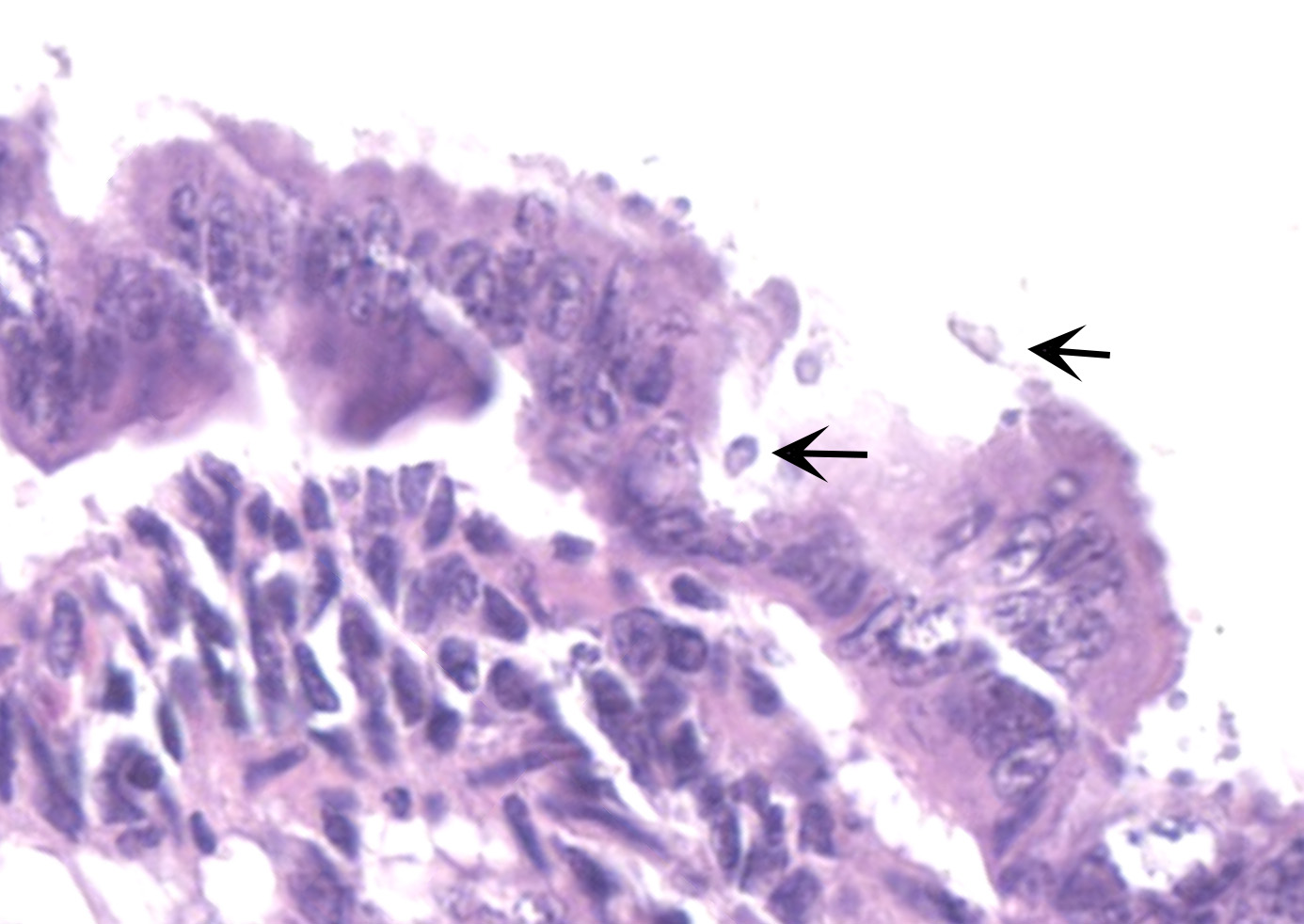
Microscopic Description:
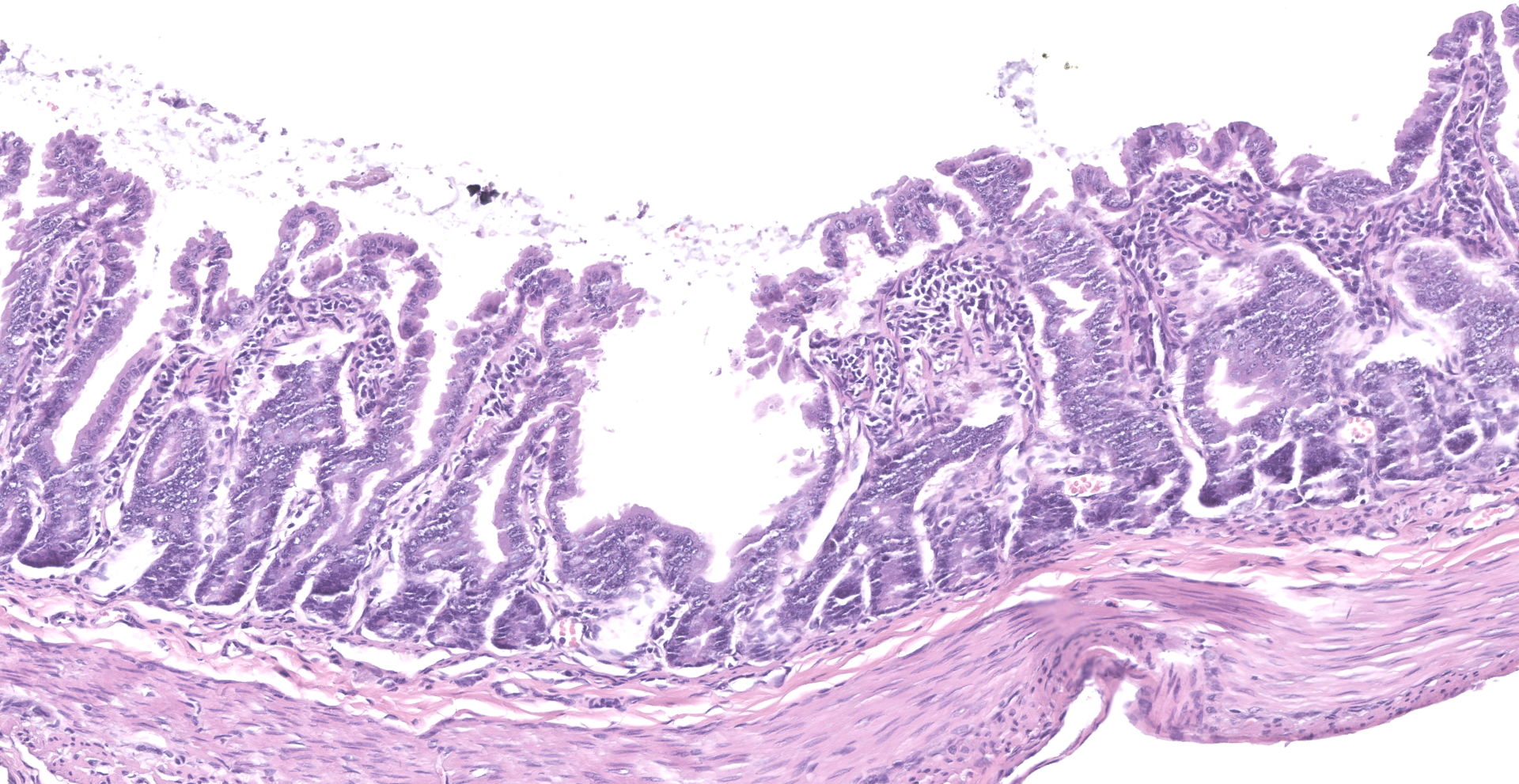
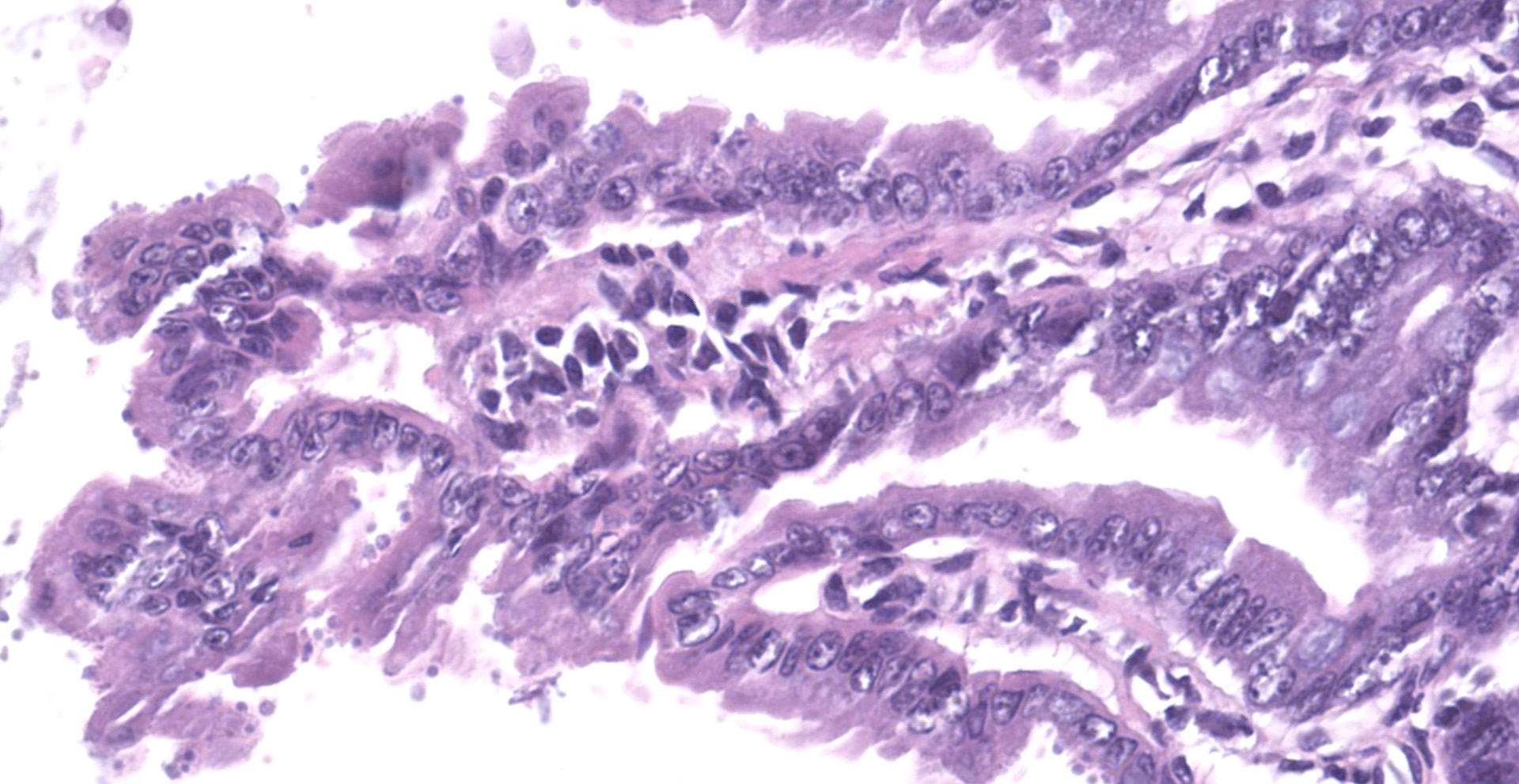
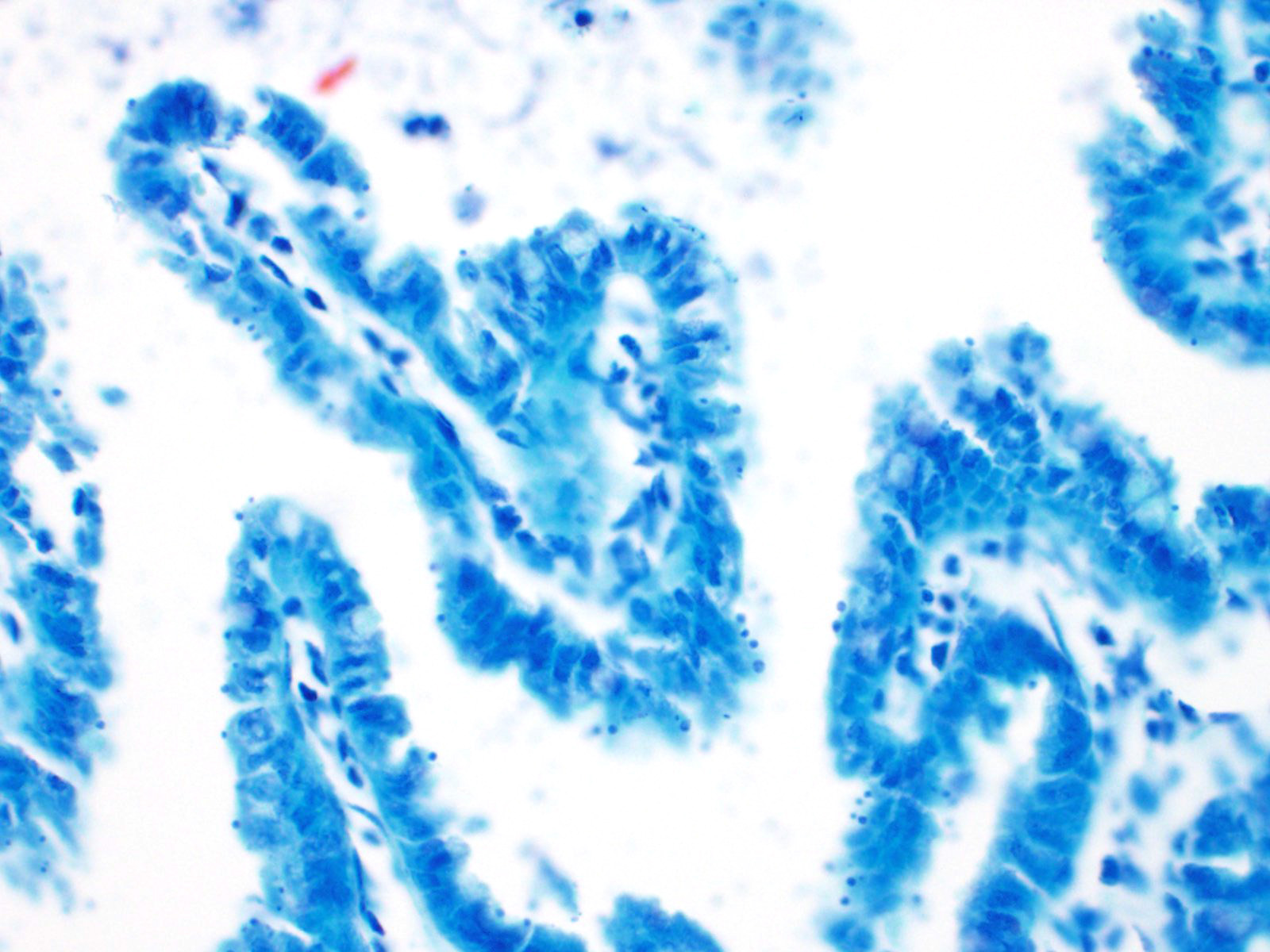
The slide consists of multiple sections of small intestine. The villi are mildly blunted with occasional fusion. The epithelial cells are minimally to mildly hypertrophied and somewhat disorganized. Adhered to the surface and within the microvilli of the epithelial cells are moderate numbers of small, 2-4 micron diameter, basophilic, spherical protozoa. A separate population of protozoa is present within the lumen (numbers vary from section to section). These organisms are piriform to crescent-shaped, measure 5-7 microns in maximum dimension, and have two internal, parallel, oval, basophilic nuclei.
Contributor's Morphologic Diagnoses:
1. Small intestine, villus blunting and fusion, multifocal, mild with epithelial cell hypertrophy and dysplasia with intralesional protozoa (morphology consistent with Cryptosporidium sp.)
2. Small intestine, intraluminal diplomonads, rare (morphology consistent with Giardia sp.)
Contributor's Comment:
This is a case of a concurrent infection of both Giardia sp. and Cryptosporidium sp. in a laboratory animal species and setting.
Cryptosporidium sp. are a group of zoonotic, protozoa of the phylum Apicomplexa. They are small, approximately 2-5 microns in diameter (depending on the life cycle stage) and are found on the epithelia of the gastrointestinal, respiratory, pancreatic, and biliary tracts of multiple animal species. There are many known species of Cryptosporidium, with some showing species specificity. The following are a few known species affecting domestic and exotic animal species: C. parvum in cattle, ruminants, and humans; C. andersoni in cattle; C. suis in pigs; C. felis in cats; C. canis in dogs; C baileyi and C. meleagridis in birds; and C. serpentis in reptiles.4,12 Transmission of the protozoa is direct via fecal-oral route and often occurs due to contaminated drinking water as well as close contact with infected animals.6 Pre-mortem diagnosis can be achieved by the rapid fecal antigen-capture ELISA or examining fresh feces microscopically.9
The life cycle of Cryptosporidium is similar to that of other Apicomplexans.4,12,14 After ingestion or inhalation of the oocyst stage, the sporozoites within the oocyst exit via excystation and adhere to mucosal epithelial cells. The sporozoites invade the cells and become surrounded by host cell membranes to form parasitophorous vacuoles. These vacuoles are often described as ?extracytoplasmic? because they communicate with the host cell cytoplasm only via a feeder organelle. Within the parasitophorous vacuoles, the sporozoites develop into trophozoites, which then undergo asexual reproduction to form first generation meronts or schizonts that contain 4-8 merozoites. These merozoites are released into the lumen, which in turn infect additional epithelial cells. Merozoites then form second generation meronts (merogony) with additional merozoites, or undergo sexual reproduction and develop into gametes (gametogomy). Male gametes are designated as microgamonts, and female gametes are known as macrogamonts. When combined, the gametes form an oocyst that then sporulates to contain four sporozoites. Once released, thin-walled sporocysts break down, released the sporozoites and allowing for autoinfection of the host. Thick-walled oocysts are passed in the feces.
Cryptosporidium sp. classically causes disease in immunocompromised animals and humans, particularly neonates and adults with underlying immunosuppressive diseases. In humans, Cryptosporidium is a very important zoonotic agent, typically affecting infants and young children as well as those with immunosuppressive conditions, most notably HIV.2 Interestingly, disease in SIV-macaques has been reported to mimic an extraintestinal disease seen in HIV infected patients.8,13 In most animal species, especially young cattle, the protozoan generally causes a malabsorptive diarrhea of varying severity as a result of damage to the villi of enterocytes and, to a lesser extent, due to a reduction in overall absorptive surface area by the organisms themselves. Furthermore, the organism can incite a variable inflammatory response in the mucosa.2 Infected marmosets may develop enterocolitis but usually remain subclinical.9 Asymptomatic animals may become carriers and continue to intermittently shed the organism.
Giardia sp. are zoonotic, diplomonads that belong to the family Hexamitidae. They are typically found in the lumen of the small intestinal tract and are one of the more common flagellates of mammals and birds.4 Unlike other members of the family (Spironucleus, Trichomonas), Giardia lack an undulating membrane ? the trophozoite form contains two nuclei, two median bodies, two axonemes, and four pairs of flagella. Currently, there are six known species that infect a wide range of animal species: G. agilis in amphibians, G. ardeae and G. pstittaci in birds, G. muris and G. microti in rodents, and G. duodenalis in mammals.10 Furthermore, Giardia duodenalis (a.k.a. Giardia lamblia or Giardia intestinalis) has several known, morphologically similar genotypes, each of which show some species specificity: assemblages A and B commonly infect humans; assemblages A and E infect hoofstock; assemblages C and D infect dogs; and assemblage F infects cats.10
The life cycle of Giardia sp. is rather simple in comparison to Cryptosporidium. There are two stages of the parasite: cyst and trophozoite.4,12 The environmentally-resistant cyst is the infective form of the parasite, and is passed in the feces. Once ingested, excyzoites are released, and transform into trophozoites, which then adhere to the microvilli of intestinal epithelial cells by an adhesive disk on the ventral surface of the organism.16 The trophozoites will then replicate and encyst, followed by passage in the feces. Transmission is direct via fecal-oral route and, like Cryptosporidium, often occurs due to contaminated food and water sources. Diagnosis is typically made by demonstrating protozoal cysts in fecal floats or trophozoites in fecal or intestinal smears. Additionally, an ELISA and immunofluorescent staining of the cyst wall can be used for detection of the organism in feces.
Disease caused by Giardia is generally asymptomatic in humans and most animal species6 and usually does not incite a significant host inflammatory response.2 However, in young dogs and cats, Giardiasis is an important cause of chronic diarrhea. Moreover, Giardia is associated with enteric disease in neonatal calves, especially those with a poor growth rate. Infected marmosets do not usually show evidence of clinical disease, but will intermittently shed the organism.9 Furthermore, Giardia may be a contributing factor to marmoset wasting syndrome.7
Contributing Institution:
Johns Hopkins University School of Medicine
Department of Molecular and Comparative Pathobiology
http://www.hopkinsmedicine.org/mcp
JPC Diagnosis:
1. Jejunum: Villar blunting and fusion, diffuse, mild to moderate with numerous intracellular apicomplexans, etiology consistent with Cryptosporidium sp.
2. Jejunum, lumen: Few extracellular flagellates, etiology consistent with Giardia sp.
3. Mesentery: Fat atrophy, diffuse, marked.
JPC Comment:
The contributor provides a thorough review of Cryptosporidium sp. and Giardia sp., two important zoonotic apicomplexan parasites encountered in many veterinary species.
Cryptosporidum sp. was first identified in laboratory mice by Tyzzer in 1907, but its role as a pathogen wasn't recognized until the 1970s when it was identified to cause disease in mammals, particularly calves. In immunocompetent mammalian hosts, cryptosporidiosis is generally a self-limiting disease. When infected, humans typically exhibit a 3-7 day incubation period followed by an acute onset of diarrhea and abdominal cramping with symptoms typically lasting 7-10 days. As noted by the contributor, cryptosporidiosis presents a serious risk to immunocompromised hosts and is one of the most serious opportunistic infections facing AIDs patients.5
Additional flagellates other than Giardia sp. that parasitize the intestinal tract include Spironucleus sp. and trichomonads. Each have distinguishing characteristics, some of which are more apparent in tissue sections whereas others are best demonstrated in fresh fecal preparations. All are similar in size, with Spironucleus sp. being the smallest (5-12µm x 2-7µm trophozoites), followed by trichomonads (8-14µm trophozoites), and Giardia sp. being the largest (10-20µm x 5-15µm trophozoites with 10-14µm x 8-10µm cysts). In fresh fecal smears, Spironucleus sp. can be identified as small piriform flagellates that lack a sucking disk. Trichomonads are also characterized by a pear shape but are larger with a pointed posterior end from which a linear axostyle may protrude, a single nucleus, 3-5 anterior flagella, and an undulating membrane contiguous with the posterior flagellum. Trichomonad trophozoites are destroyed by floatation solutions but can be identified in preparations using saline. In tissue sections, Giardia sp. are often closely opposed to the mucosal epithelium with a ventrally flattened appearance due to the ventral adhesive disk, which is a specialized feature allowing for close association to the mucosal epithelium. In contrast, trichomonads have a rounder profile without ventral flattening in association with the small intestine mucosal epithelium, lack a cyst stage, have a single nucleus, and an undulating membrane. Centrifugation floatation using 33% ZnSO4 is preferred over fresh feces for the detection of Giardia sp. cysts. In addition, Giardia sp. trophozoites have a characteristic ?falling leaf? or wobbling swimming motion in fresh fecal smears, which aids in distinguishing them from trichomonads.11
Although marmosets (Callithrix sp.) have a long history of being kept as pets, biomedical research saw a dramatic rise in their utilization following the establishment of the National Institutes of Health (NIH) primate centers program in the early 1960s. Multiple factors make these New World primates ideal models for biomedical research, including an approximately 93% sequence identity to the human genome (similar to macaques), small size (adults typically weight 300-450g), relatively short lifespan in comparison to other primates (13 years on average), and they are the most fertile of the primates, reaching sexual maturity by 1.5 years of age and produce litters of 2-3 offspring every 5-6 months. Marmosets are particularly well suited for cognition, behavior, and mental illness research studies given their shared functional brain architecture and behaviors with humans. Unlike Old World monkeys (OWM), marmosets have a lissencephalic (smooth) cortex, which greatly facilitates the recording and manipulation of brain activity in comparison to their OWM counterparts. Susceptible to a number of viruses, including hepatitis C, dengue, herpesvirus, SARS, Marburg, and Ebola, marmosets are ideal models for pharmacology and toxicology studies, especially since smaller amounts of test compounds are required due to their small body size. The most commonly used species is the common marmoset (C. jacchus), in addition to black-tufted and white-headed or Geoffroy's marmosets.3
One of the most significant problems facing captive marmoset colonies is 'wasting marmoset syndrome' (WMS), which affects 28-60% of captive marmosets and is involved in 50-80% of deaths. Multiple reports state the primary disease of WMS is an inflammatory bowel disease (IBD) with chronic enteritis.14 Common symptoms include weight loss, decreased muscle mass, alopecia (commonly at the tail base), and chronic diarrhea. One study found most marmosets weighing less than 325g were affected by WMS and 100% of affected marmosets suffered chronic weight loss of 0.05% of the peak body weight per day.1
Common bloodwork abnormalities associated with WMS include anemia, hyperproteinemia, hypoalbuminemia, and elevated serum alkaline phosphatase. However, post-mortem examination is the gold standard for the diagnosis of WMS, with the hallmark lesion being chronic lymphoplasmacytic enterocolitis. The underlying cause of WMS is unknown with proposed etiologies including food allergies, parasitism, and autoimmune disease. WMS can significantly disrupt research studies and breeding programs as affected animals are often not identified until late in the disease process.1
One study found matrix metalloproteinase 9 (MMP9) serum levels are be significantly elevated in marmosets affected by WMS. This enzyme is thought to play a role in the pathogenesis of similar inflammatory conditions, including human and murine inflammatory bowel disease, by degrading extracellular matrix components. Therefore MMP9 may not only be useful as a biomarker for WMS diagnosis but also as a target of therapeutic intervention.13 A more recent study found marmosets with WMS treated with transexamic acid, in combination with amino acid and iron supplementation, to have reduced serum MMP9 levels, ameliorated alopecia, and increased body weight and hematocrit. These animals continued to gain or maintain body weight for at least three months following the experiment. Transexmanic acid suppresses MMP9 by inhibiting its activator, plasmin.15
References:
1. Baxter VK, Shaw GC, Sotuyo NP, et al. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One. 2013;8(12):e82747.
2. Certad G, Viscogliosi E, Chabe M, Caccio SM. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017. (Epub ahead of print).
3. Colman RJ, Capuano S, Bakker J, Keeley J, Nakamura K, Ross C. Marmosets: Welfare, Ethical Use, and IACUC/Regulatory Considerations [published online ahead of print, 2021 Feb 23]. ILAR J. 2021;ilab003.
4. Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues. 2nd ed. Washington, DC: Armed Forces Institute of Pathology; 1984:6-7, 37-39.
5. Hahn NE, Capuano SV 3rd. Successful treatment of cryptosporidiosis in 2 common marmosets (Callithrix jacchus) by using paromomycin. J Am Assoc Lab Anim Sci. 2010;49(6):873-875.
6. Johnson-Delaney CA. Parasites of captive nonhuman primates. Vet Clin Exot Anim. 2009;12(3):563-581.
7. Kramer JA, Hachey AM, Wachtman LM Mansfield KG. Treatment of Giardiasis in common marmosets (Callithrix jacchus) with Tinidazole. Comp Med. 2009;59(2): 174-179.
8. Kvac M, McEnvoy J, Stenger B, Clark M. Cryptosporidiosis in other vertebrates. In: Caccio SM and Widmer G, ed. Cryptosporidium: parasite and disease. New York: Springer; 2014: 237-323.
9. Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med. 2003;53(4):369-382.
10. Ryan U, Caccio SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43:943-956.
11. Sheppard BJ, Stockdale Walden HD, Kondo H. Syrian hamsters (Mesocricetus auratus) with simultaneous intestinal Giardia sp., Spironucleus sp., and trichomonad infections. J Vet Diagn Invest. 2013;25(6):785-790.
12. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier; 2016:293-243.
13. Yanai T, Chalifoux LV, Mansfield KG, Lackner AA, Simon MA. Pulmonary Cryptosporidiosis in Simian immunodeficiency virus-infected Rhesus Macaques. Vet Pathol. 2000;37(5):472-475.
14. Yoshimoto T, Niimi K, Takahashi E. Serum matrix metalloproteinase 9 (MMP9) as a biochemical marker for wasting marmoset syndrome. J Vet Med Sci. 2016;78(5):837-843.
15. Yoshimoto T, Niimi K, Takahashi E. Tranexamic Acid and Supportive Measures to Treat Wasting Marmoset Syndrome. Comp Med. 2016;66(6):468-473.
16. Zachary JF. Protozoan diseases of organ systems. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017:236-238.