Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 23
April 25th, 2018
CASE IV: 109234-16 (JPC 4100936).
Signalment: 15-week-old female intact Boston terrier (Canis familiaris), canine.
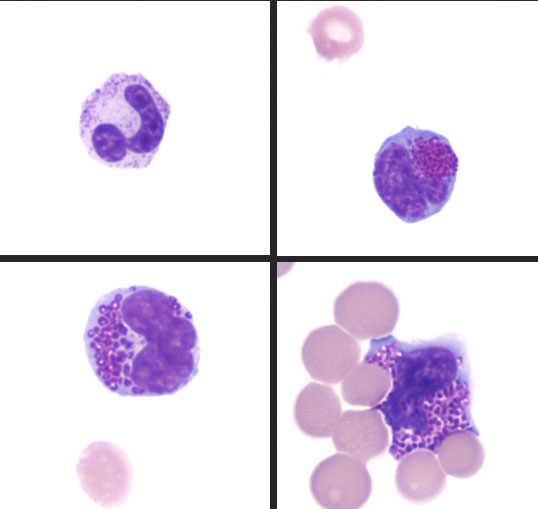
History: A 15-week-old intact female Boston Terrier was presented to the Neurology Service of the Cornell University Hospital for Animals with a 7-week history of hyporexia, dysphagia, difficulty ambulating, failure to gain weight, and lethargy. The physical examination revealed dull mentation, poor body condition, non-ambulatory tetraparesis, severe joint laxity, and bilateral corneal opacities. MRI and CT imaging showed evidence of skull malformation, hydrocephalus, cerebral atrophy, severe vertebral and intervertebral disc malformations, and epiphyseal dysplasia. Cytology of the blood, cerebrospinal fluid, and joint fluid revealed cytoplasmic vacuolation and metachromatic cytoplasmic granules in neutrophils, lymphocytes, and macrophages. Based on these findings a presumptive diagnosis of mucopolysaccharidosis (MPS) was made and the owner elected euthanasia due to the poor prognosis.
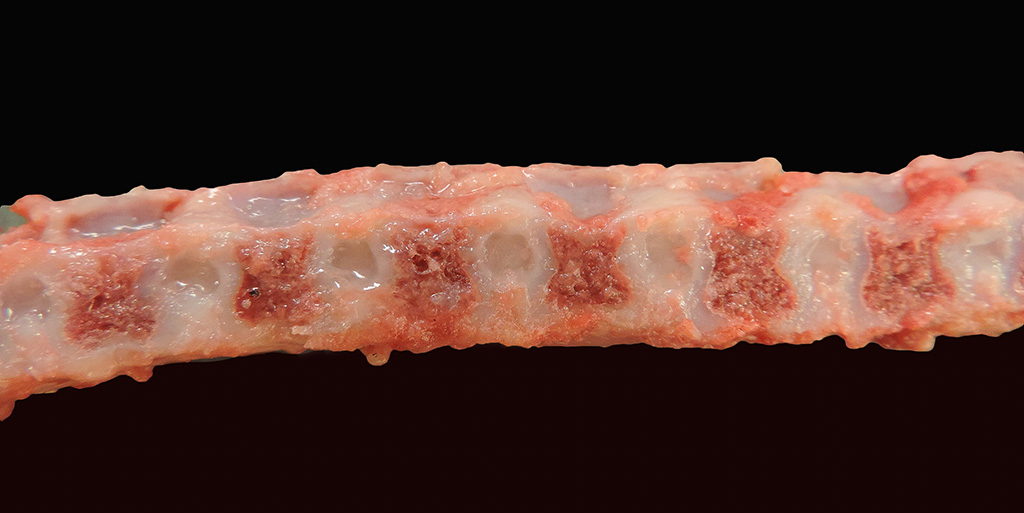
Gross Pathology: Within both corneas were 0.4 cm in diameter, irregularly shaped areas of white-blue opacity. The left and right 10th, 11th, and 12th ribs were widened, flattened, and bulging laterally and ventrally. All four limbs had severe laxity of all of their joints and the left forelimb was externally rotated at the carpus. On the ventral midline at the level of the umbilicus was a small, 0.6 cm in diameter, focal, round, soft, reducible, protrusion (umbilical hernia).
Bilaterally, the proximal epiphyseal plates of the femur and humerus and distal epiphyseal plate of the radius and ulna were displaced and irregular (epiphyseal dysplasia). All vertebral bodies were shortened with thinning of the cortex and an approximate 1:1 ratio of the vertebra to intervertebral disc length. The intervertebral discs were rounded, widened, and hollow at the center and contained clear, loose, gelatinous material (dysplastic nucleus pulposus). The rostral mandible at the level of the symphysis was widened and thickened up to 3.0 x 3.0 x 1.0 cm. The xiphoid process extended 3.0 cm caudal to the last sternebra. On the dorsal surface of both carpi and arising between the carpal bones were five (right) and four (left) raised, up to 0.9 x 0.5 x 0.3 cm, well-demarcated, tan to pink, soft, fluctuant, cystic structures continuous with the joint capsules. These cystic structures contained <0.5 mL of slightly opaque, red-tinged fluid. The joints of all four limbs contained mildly increased amounts of cloudy, red-tinged fluid and the joint capsules had increased elasticity.
The heart weighed 26.0 g. The leaflet of the tricuspid valve along the interventricular septum was glistening, smooth, and focally thickened up to 1.0 x 0.6 x 0.2 cm. The mitral valve had seven similar, nodular thickenings up to 0.3 x 0.1 x 0.1 cm.
The meninges were diffusely opaque and moderately thickened. Sectioning of the brain revealed a mild dilation of the lateral ventricles (hydrocephalus).
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
Berry urine MPS spot test: Positive
Microscopic Description:
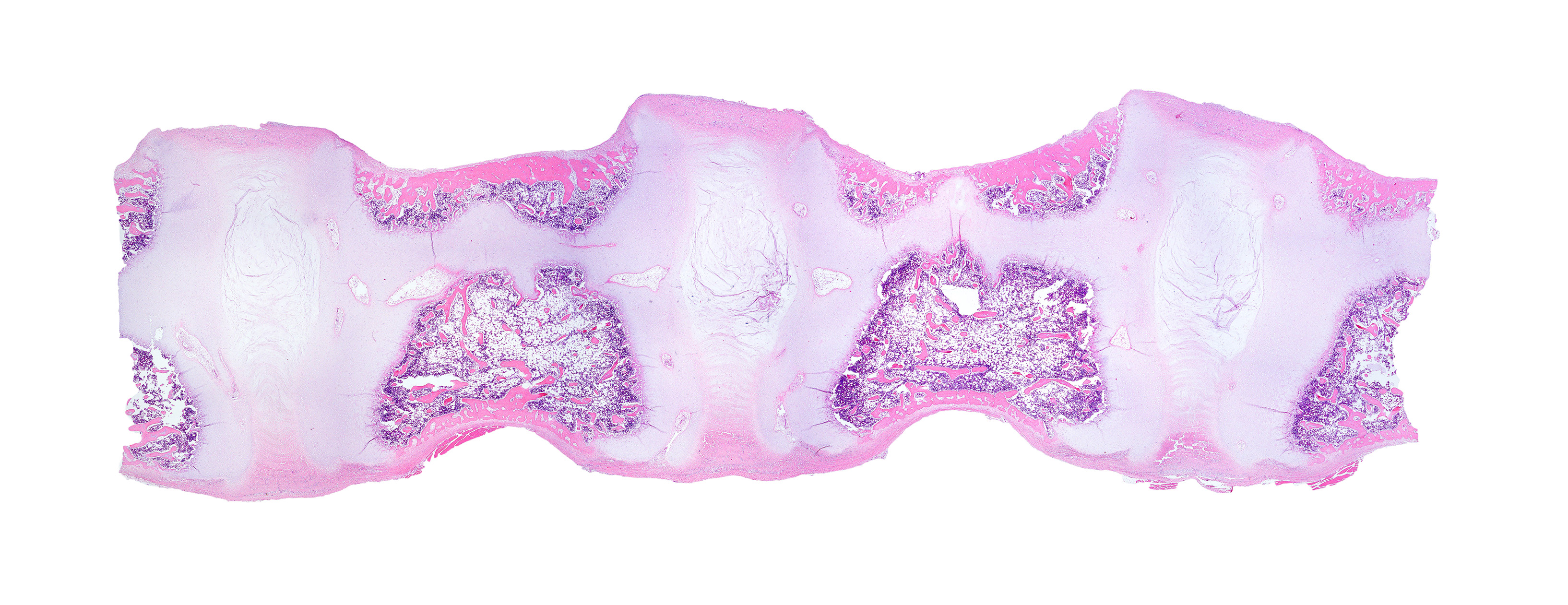
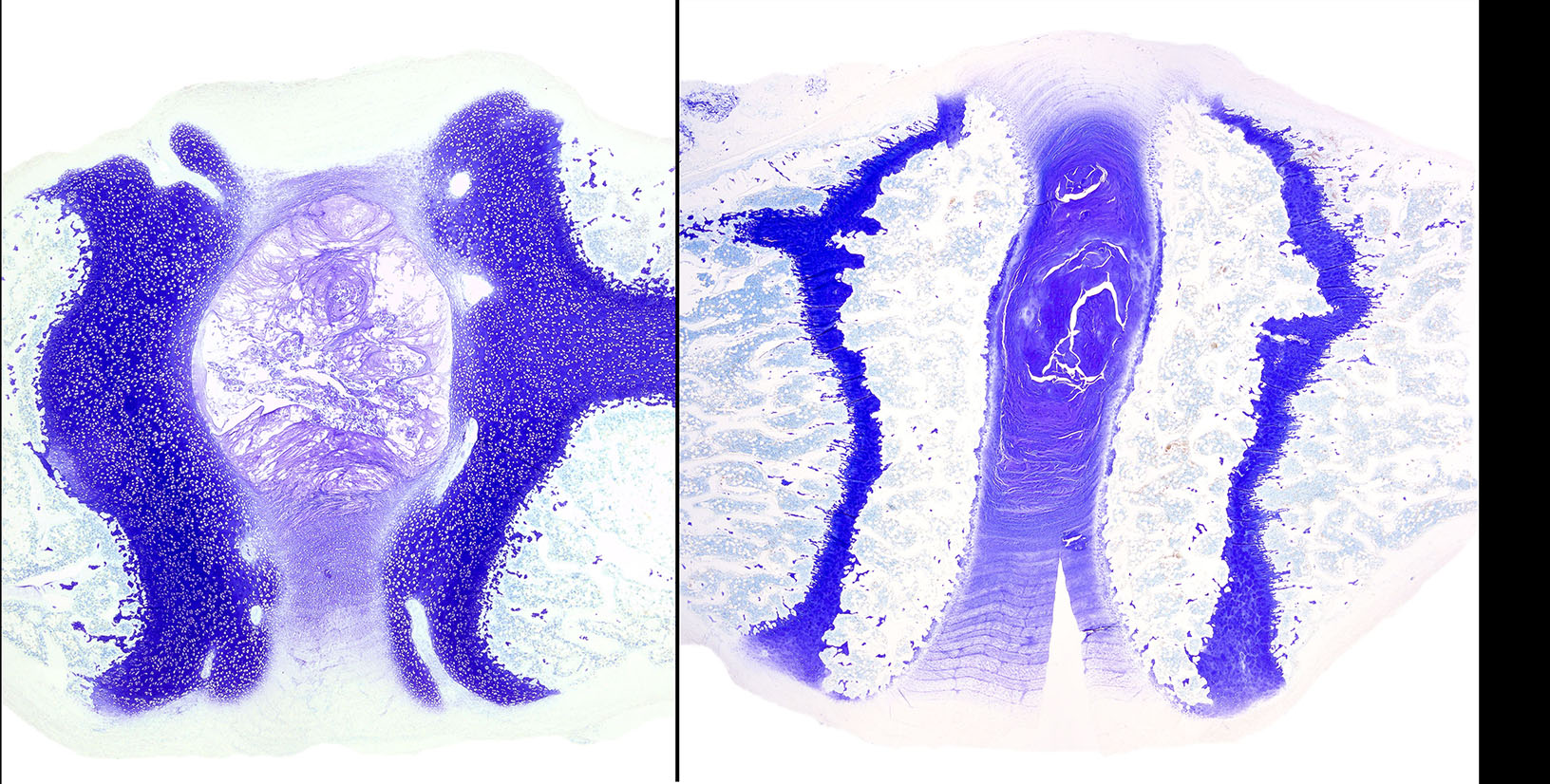
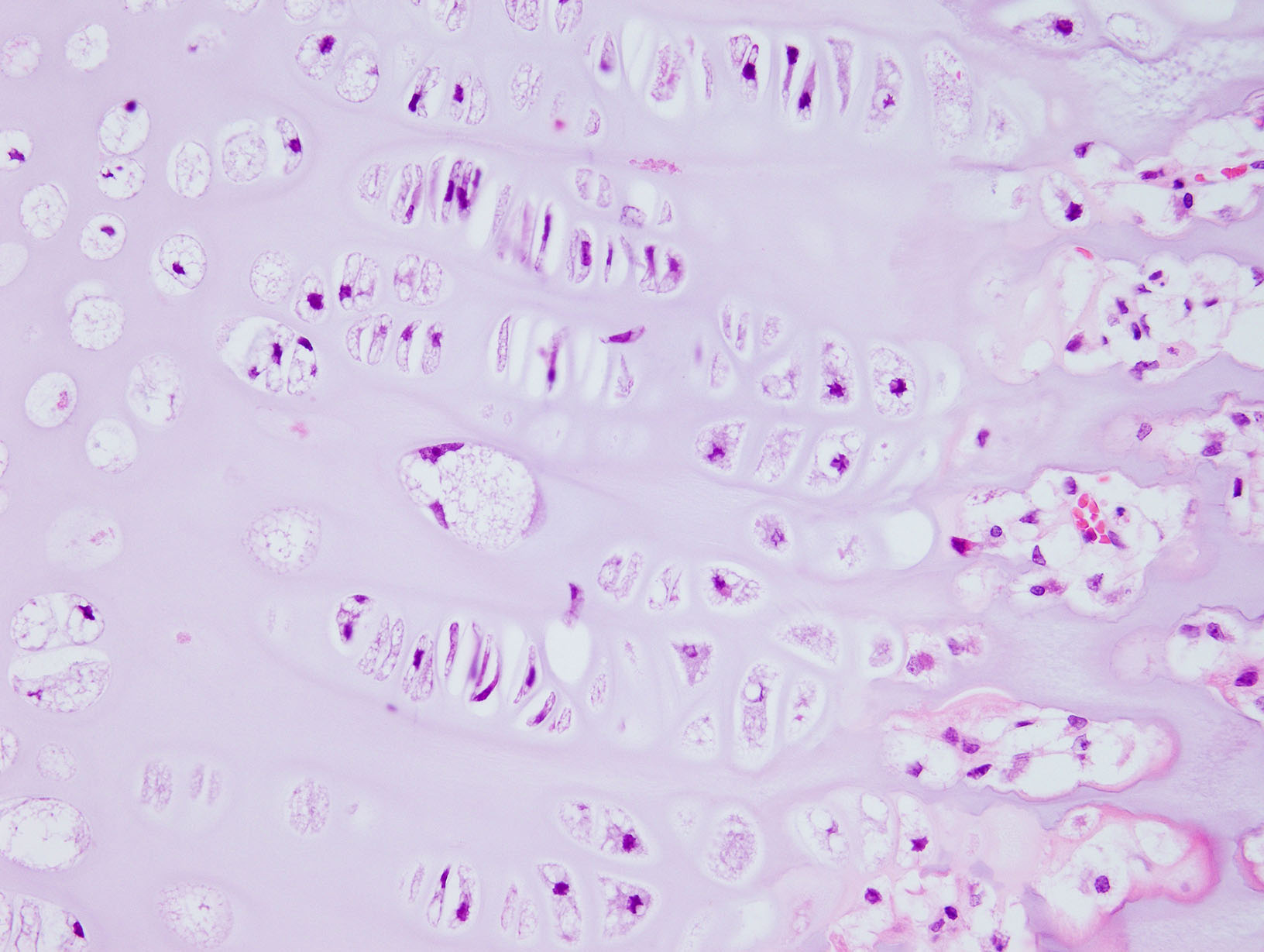
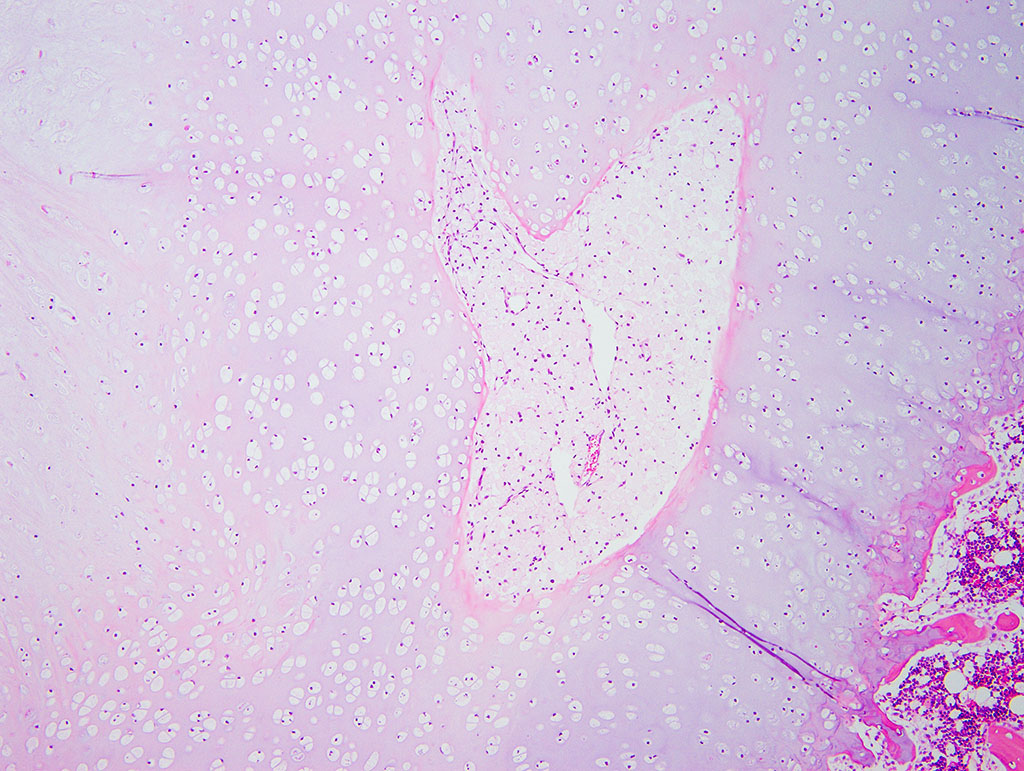
Diffusely, the epiphyseal cartilage is severely thickened with an irregular contour and lacking secondary centers of ossification. Multifocally within, and occasionally crossing the epiphyseal cartilage, are broad tracts that lack cartilage with small blood vessels surrounded by abundant large polygonal cells with foamy cytoplasm and eccentric nuclei (transphyseal vessels). Consistently bridging the medullary space between epiphyseal cartilage are broad columns of cartilage with occasional extensions into the medullary space ventrally and dorsally. Chondrocytes are mildly enlarged, rounded, and have finely vacuolated cytoplasm and are occasionally binucleated. At the chondro-osseous junction is a narrow proliferative and hypertrophic zone with mineralized cartilage and a paucity of osteoclasts. Trabecular bone is sparse and discontinuous with retained cartilage cores. The nucleus pulposus is composed of abundant wispy to frothy basophilic matrix, small numbers of scattered 2 to 4 µm in diameter round, eosinophilic globules, and moderate numbers of large polygonal cells with vacuolated cytoplasm and eccentric nuclei (variable severity between sections). Expanding the collagen fiber matrix of the ventral and dorsal longitudinal ligaments are large numbers of individual and clustered large polygonal cells with foamy cytoplasm and eccentric nuclei.
Contributor’s Morphologic Diagnosis:
Lumbar vertebrae:
- Chondrodysplasia
- Epiphyseal dysplasia with loss of secondary site of ossification
- Intervertebral disc dysplasia
- Osteopenia
Contributor’s Comment: The combined clinicopathologic, gross, and histologic findings of this puppy were strongly suggestive of mucopolysaccharidosis (MPS) with features that overlap with other reported cases of MPS in dogs. To confirm the diagnosis, urine was submitted to PennGenn Laboratories at the University of Pennsylvania for a urinary Berry MPS spot test3. In addition, a severe deficiency of β-glucuorindase was detected in the serum (personal communication, Dr. Urs Giger). These results were consistent with a Type VII MPS, also known as Sly syndrome in humans. Unfortunately, insufficient samples were available to determine the underlying genetic mutation in this case.
Mucopolysaccharidoses are a group of related lysosomal storage diseases that are the result of genetic deficiencies of key enzymes involved in the normal degradation of mucopolysaccharides. Mucopolysaccharides are glycosaminoglycans; long-chain carbohydrates attached to protein cores that are commonly found in the ground substance of connective tissues throughout the body. Examples of glycosaminoglycans include dermatan sulfate, heparin sulfate, keratin sulfate, and chondroitin sulfate. MPS disorders are broken down into types designated by the underlying enzyme deficiency. For example, Type I MPS is the result of a deficiency in the α-L-iduronidase enzyme and results in the accumulation of dermatan sulfate and heparin sulfate within lysosomes and in the urine. Thus far, only MPS types I, II, IIIA, IIIB, IIIC, IIID, VI, and VII have been identified in domestic animals.
Lysosomes are small organelles within the cytoplasm that are responsible for the breakdown of endogenous and exogenous materials, including byproducts of normal cellular processes9. When deficiencies of specific lysosomal acid hydrolases or components of enzyme trafficking occur, substrates are not properly catabolized and can accumulate within the lysosomes. This accumulation of the substrate is characteristic of lysosomal storage diseases, a heterogeneous group of disorders with a common feature of accumulating metabolites. The buildup of this material can cause lysosomes to become large and hinder normal cellular processes and may even lead to cell death. Errors in lysosomal processing can also result in the accumulation of autophagic substrate during normal cell turnover which can cause altered cellular metabolism and result in apoptosis. Long-lived postmitotic cells, fixed and mobile macrophages, and the cells most active in metabolizing these substrates are most susceptible to storage disease.
The lesions associated with MPS are related to the distribution and functions of the glycosaminoglycans affected by the enzyme deficiency. The key gross findings of this case that are consistent with MPS include skeletal deformities with shortened vertebral bodies, incomplete ossification of the vertebral end plates with subsequent widening of the intervertebral disc spaces, deformed epiphyses of the appendicular skeleton, facial dysmorphism, nodular thickening of the atrioventricular valves, meningeal thickening and opacity, and bilateral corneal opacities. The secondary ossification center of the distal epiphysis of the tibia, for example, is expected to be developed by 10-30 days of age but was completely lacking in this dog5. In addition to the vacuolation of chondrocytes, macrophages, and other stromal cells in the vertebral column, vacuolation in the Purkinje cells of the cerebellum, the stromal cells and macrophages of the aorta and trachea, proximal convoluted tubular epithelial cells, and synovium of appendicular joints were also seen in this case. The vacuolation of the epithelial cells of the kidneys is thought to reflect elevated levels of circulating glycosaminoglycans in the blood that are excreted through the glomerulus and reabsorbed in the renal tubular epithelium11. Surprisingly, vacuolation of Kupffer cells and hepatocytes was not a feature in this case despite being reported in another case of type VII MPS6.
One of the early clues of MPS in this case was the presence of metachromatic intracytoplasmic granules (Alder-Reilly bodies) in neutrophils, lymphocytes, and macrophages in peripheral blood, joint fluid, and cerebrospinal fluid. This is a common finding in cases of MPS and is best observed with Leishman or Wright-Giemsa stains as Diff-Quick-stained smears do not reveal inclusions as readily in some reports8.
Histochemical staining of the vacuoles was variable with Alcian blue and periodic acid-Schiff; however, it is suspected that the inclusion material can be lost during processing. In this case, the only cells highlighted with Alcian blue stain were within the wall of the aorta. Some reports have suggested that better success is obtained by using these stains on frozen tissues12. Electron microscopy could reveal dilated lysosomes that appear empty (electron-lucent), electron dense, or contain lamellar swirls (zebra bodies). Electron microscopy was not performed in this case.
JPC Diagnosis: Vertebral column: Chondrodysplasia, diffuse, severe with epiphyseal dysplasia, loss of secondary ossification sites, intervertebral disc dysplasia, and chondrocytic, fibroblastic and histiocytic vacuolation, Boston terrier (Canis familiaris), canine.
Conference Comment: Mucopolysaccharidosis is aptly described by the contributor above.
Table 1: Selected mucopolysaccharidoses in domestic animals 5,11,13,14
|
Disease |
Storage product(s) |
Deficient enzyme(s) |
Species |
Breed(s) |
|
MPS type I “Hurler’s disease” |
Different glycosaminoglycans (dermatan sulfate, heparan sulfate, keratin sulfate, chondroitin sulfate) |
α-L-iduronidase |
Dog, cat |
Plott hound Domestic shorthair |
|
MPS type II “Hunter syndrome” |
Iduronate-2-sulfatase |
Dog |
Labrador retriever (1) |
|
|
MPS type III A, B, D “Sanfilippo syndrome” |
Heparan sulfate sulfamidase (SGSH; MPS type IIIA), a-N-acetylglucosaminidase (NAGLU; MPS type IIIB), and N-acetylglucosamine-6-sulfatase (GNS; MPS type IIID) |
Dog, cattle, goat |
IIIA – Dachshund, New Zealand Huntaway dogs IIIB – Schipperke dogs, cattle, emus IIID – goats |
|
|
MPS type VI “Maroteaux-Lamy disease” |
ARSB (arylsulfatase B) |
Dog, cat |
Miniature Pinscher, Miniature Schnauzer, Toy Poodle, Welsh Corgi, Chesapeake Bay Retriever, Great Dane Domestic shorthair, Siamese |
|
|
MPS type VII “Sly disease” |
Β-glucuronidase |
Dog, cat |
German Shepherd Dog Domestic shorthair |
During the conference the moderator noted that the cartilage cores are not running parallel to the axis of the bone but perpendicular, indicating infractions or microfractures in the bone. In general, the bone is weakly mineralized with few primary trabeculae being formed and a disorganized zone of hypertrophy, indicative of a developmental lesion.
Contributing Institution:Cornell University
240 Farrier Road
Ithaca, NY 14853
http://www.vet.cornell.edu/biosci/pathology/
References:
- Abreu SJ, Hayden P, Berthold IM, Shapiro S, Decker D, Haskins M. Growth plate pathology in feline mucopolysaccharidosis VI. Calcified Tissue International Calcif Tissue Int. 1995; 57(3):185-190.
- Berry HK. Screening for mucopolysaccharide disorders with the Berry spot test. Clinical Biochemistry. 1987; 20:365-371.
- Burbidge HM, Thompson KC, Hodge H. Post Natal development of canine caudal cervical vertebrae. Research in Veterinary Science. 1995; 59(1):35-40.
- Canine and Feline Epiphyseal Plate Closure and Appearance of Ossification Centers. http://cal.vet.upenn.edu/projects/saortho/appendix_c/appc.htm
- Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 1. 6th ed. St. Louis, MO: Elsevier; 2016:289-290.
- Dombrowski DC, Silverstein K, Carmichael P, Wang P, O'Malley TM, Haskins ME, Giger U. Mucopolysaccharidosis type VII in a German shepherd dog. Journal of the American Veterinary Medical Association. 2004; 224(4):553-57.
- Gitzelmann R, Bosshard NU, Superti-Furga A, Spycher MA, et al. Feline mucopolysaccharidosis VII due to β-glucuronidase deficiency. Veterinary Pathology. 1994; 31:435-443.
- Jolly RD, Hopwood JJ, Marshall NR, Jenkins KS, Thompson DJ, Dittmer KE, Thompson JC, Fedele AO, Raj K, Giger U. Mucopolysaccharidosis type VI in a miniature poodle-type dog caused by a deletion in the arylsulphatase B gene. New Zealand Veterinary Journal. 2012; 60(3):183-188.
- Kumar V, Abbas AK, Aster JC. Pathologic Basis of Disease. Philadelphia, PA: Elsevier-Saunders, 2015.
- Mehta AB, Beck M, Sunder-Plassmann G. Fabry Disease: Perspectives from 5 Years of FOS. Ch. 6, Table 1.
- Palmieri C, Giger U, Wang P, Pizarro M, Shivaprasad HL. Pathological and biochemical studies of mucopolysaccharidosis Type IIIB (Sanfilippo syndrome type B) in juvenile emus (Dromaius novaehollandiae). Veterinary Pathology. 2014; 52(1):160-169.
- Shull RM, Helman, RG, Spellacy E, Constantopoulos G, et al. Morphologic and Biochemical Studies of Canine Mucopolysaccharidosis I. American Journal of Veterinary Pathology. 1984; 114(3):487-495.
- Wang P, Margolis C, Lin G, Buza EL, et al. Mucopolysaccharidosis type VI in a Great Dane caused by a nonsense mutation in the ARSB gene. Vet Pathol. 2018; 55(2):286-293.
- Wilkerson MJ, Lewis DC, Marks SL, Prieur DJ. Clinical and morphologic features of mucopolysaccharidosis type II in a dog: naturally occurring model of Hunter syndrome. Vet Pathol. 1998; 35(3):230-233.
- Yogalingam G, Pollard T, Gliddon B, Jolly RD, Hopwood. Identification of a mutation causing mucopolysaccharidosis type IIIA in a New Zealand huntaway dogs. 2002; 79(2):150-153.