Signalment:
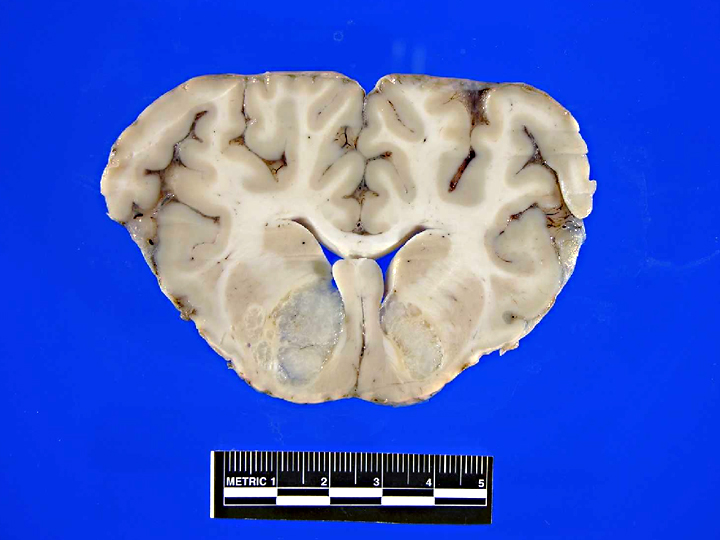
Gross Description:
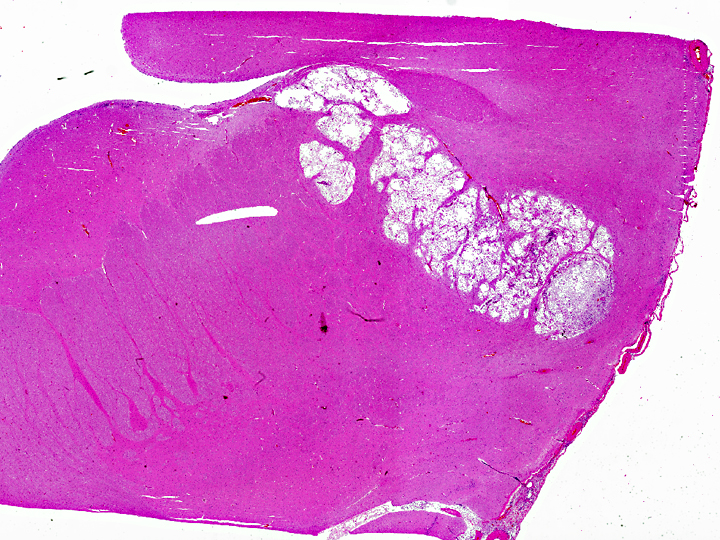
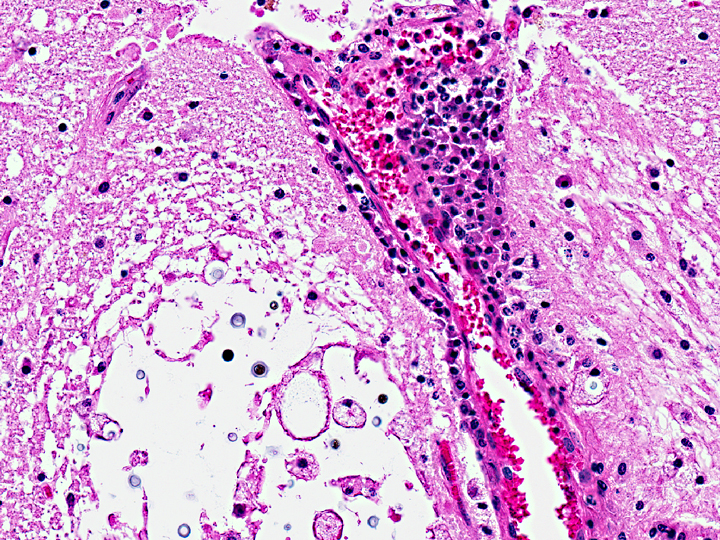
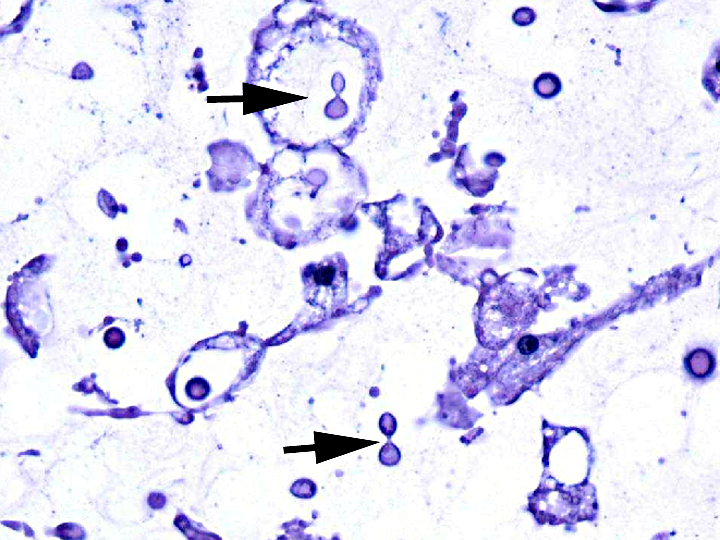
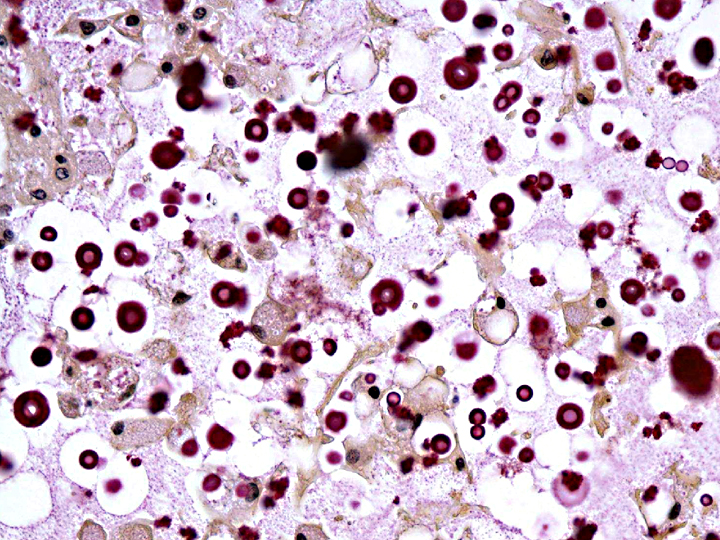
Histopathologic Description:
Sections of liver (not submitted) had mild centrilobular fatty degeneration of hepatocytes. No lesions were identified in other structures of the head.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Cryptococcal disease is neither contagious nor zoonotic; animals and people acquire the infection by inhaling infectious particles from the environment. C. neoformans is found in soil and bird droppings, especially from pigeons(4). C. gattiiis found in association with eucalyptus trees and recently in the coastal Douglas fir and western hemlock forests of British Columbia and the Pacific Northwest US(6). The infectious particle is likely the basidiospore, although dehydrated yeast bodies may also be infectious(1). C. neoformans has emerged as an important secondary infection in people with HIV/AIDS; while C. gattii also causes disease in immunocompromised hosts, cryptococcosis in previously healthy individuals is more likely to be caused by C. gattii. Cryptococcosis in cats, the most common veterinary species affected, is rarely related to documented immunodeficiency(4). Respiratory infections are the most common manifestation of disease, although systemic spread, especially to the brain, is not unusual. Some reports suggest that, in people, C. gattii infection is more likely to result in neurologic disease than is infection by C. neoformans(1, 5).
Cryptococcal virulence factors probably evolved as protection against ingestion by environmental amoeba; mammalian infection is likely accidental(1). Those virulence factors include the ability to grow at temperatures above 30o C and the distinctive large, polysaccharide capsule which protects against phagocytosis and killing by neutrophils. Production of melanin by both pathogenic species protects the organisms against oxidative damage. Although originally thought to be restricted to the eucalyptus forests of Australia, Southeast Asia and other tropical regions, C. gattii was identified in animal cases of cryptococcosis on Vancouver Island in 2000 and subsequently spread to people and animals in British Columbia, Washington and Oregon(2). Retrospective studies suggested that C. gattii may have circulated in Southern California for much longer. The mechanism of the switch from tropical to temperate climates is unknown. However, most eucalyptus associated outbreaks in Australia are of molecular type VGI, whereas 90% of isolates from the Pacific Northwest are type VGIIa, and southern California isolates are type VGIII, the genotype commonly identified in Mexico. This suggests that different genotypes have different biogeoclimatic distributions(5).
This case of neurologic cryptococcosis caused by C. gattii in a juvenile elk in Yakima County, WA represents further evidence of the spread of C. gattii infection within the Pacific Northwest region of the United States. A previous report of a case or cases of C. gattii infection in Yakima County provides no details(5). There is limited information on C. gattii infection in wildlife. In 2006, a culture survey of wildlife species on Vancouver Island and lower mainland British Columbia found a 2% positive rate in nasal cavity swabs, a rate similar to that of sampled domesticated species(6). Although no similar survey of wildlife in the Pacific Northwest is published, we would expect additional cases of C. gattii disease in wildlife in our area in the future.
JPC Diagnosis:
Conference Comment:
Urease expression by Cryptococcus spp. appears to promote sequestration in microcapillaries, which is a critical step in dissemination to the brain. The organisms predilection for the central nervous system may be partially attributed to the lack of alternative pathway complement components in cerebrospinal fluid, which would otherwise bind to the carbohydrate capsule and enhance phagocytosis and killing by neutrophils(3).
Protective immunity against Cryptococcus spp. requires a Th1 pattern of cytokine response, to include interleukin- 12 (IL-12), IL-18, interferon gamma, tumor necrosis factor beta, granulocyte-macrophage colony stimulating factor, macrophage inflammatory protein-1, and monocyte chemoattractant protein-1. Humoral factors, such as opsonization by antibody and complement are important in clearance(3).
References:
2.Byrnes III, EJ, Marr KA. The outbreak of Cryptococcus gattii in western North America: Epidemiology and clinical issues. Curr Infect Dis Rep 13: 256-261, 2011.
3.Carroll SF, Guillot L, Qureshi ST. Mammalian model hosts of cryptococcal infection. Comp Med. 2007 Feb;57(1):9-17.
4.Caswell JL. Williams KJ. The respiratory system In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, fifth edition, ed. Maxie, MG, pp.642-643, Elsiever, Edinburgh, 2007.
5.Datta K, Bartlett KH, Baer R, et al. Spread of Cryptococcus gattii into the Pacific Northwest region of the United States. Emerging Infect Dis 15:1185-1191, 2009.
6. Duncan C, Schwantje H, Stephen C, et al. Cryptococcus gattii in wildlife of Vancouver Island, Bristish, Columbia, Canada. J Wildlife Dis 42:175-178, 2006.