WSC 22-23
Conference 3
CASE II:
Signalment:
18-month-old male, Apoetm1Unc KO on C57BL/6 background (Mus musculus)
History:
This mouse was part of an atherosclerosis study and was transitioned to a Western high fat diet at 3-months of age. At 15-months, the mouse became pruritic and developed ulcerative dermatitis that wax and waned despite treatment. Humane euthanasia was elected at 18-months.
Gross Pathology:
The mouse was overly conditioned with a body condition score of 5/5. On external examination, there were deep skin erosions and ulcers on the right flank and left right forelimb that ranged in size from 1-4mm in diameter. The liver was diffusely enlarged and friable. The submandibular and renal lymph nodes were enlarged. The remainder of the postmortem examination was within normal limits.
Laboratory Results:
No laboratory findings reported.
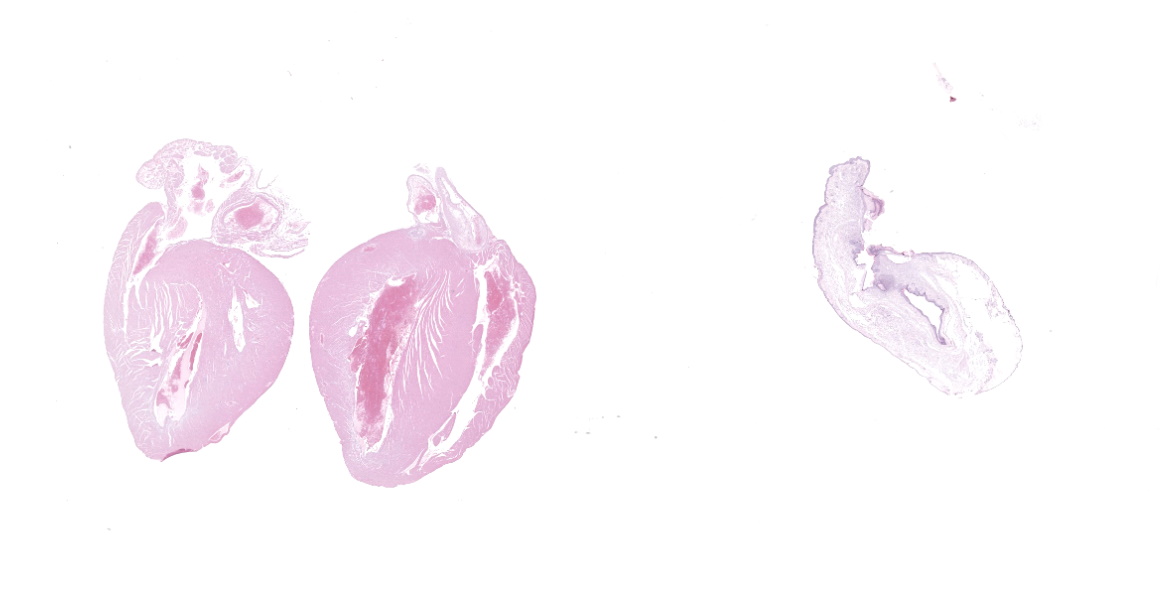
Microscopic Description:
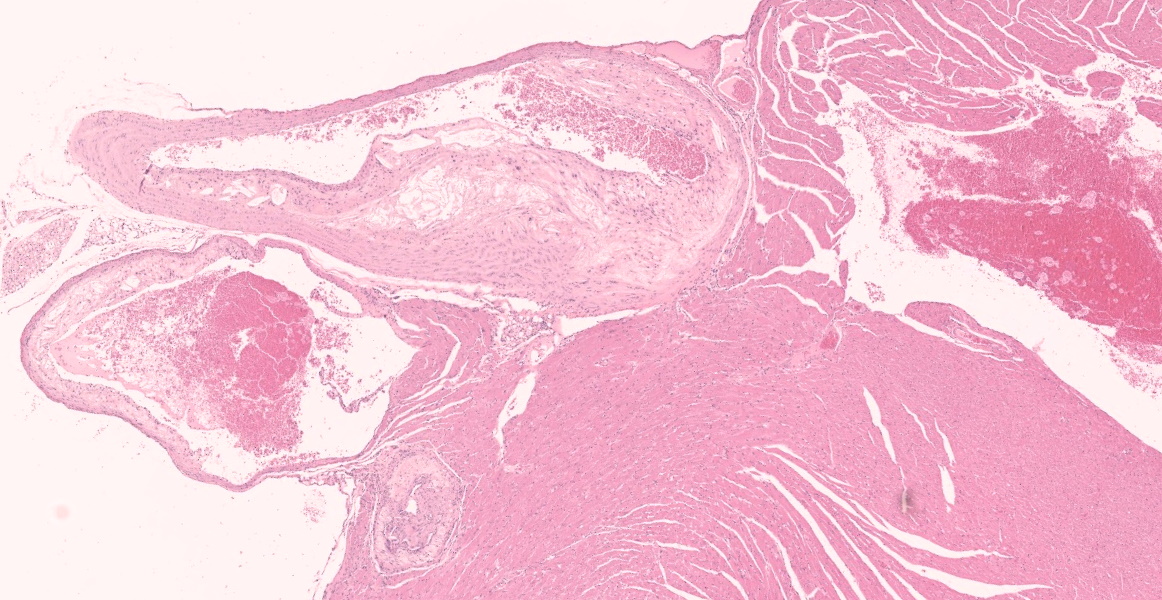
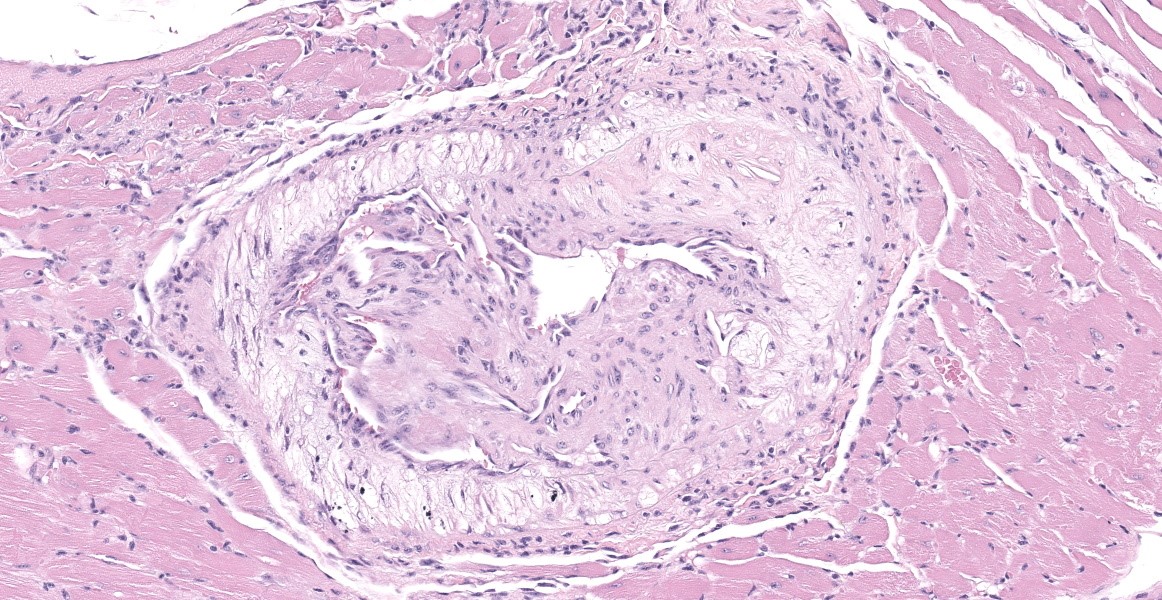
The aorta and pulmonary artery are segmentally narrowed due to luminal expansion by atherosclerotic subintimal plaques. Subintimal plaques occasionally extend into the tunica media and are composed of diffuse aggregates of acicular cholesterol cleft, plump foamy macrophages, multinucleated giant cells, and lymphocytes. There are rare foci of dystrophic mineralization and fibrosis.
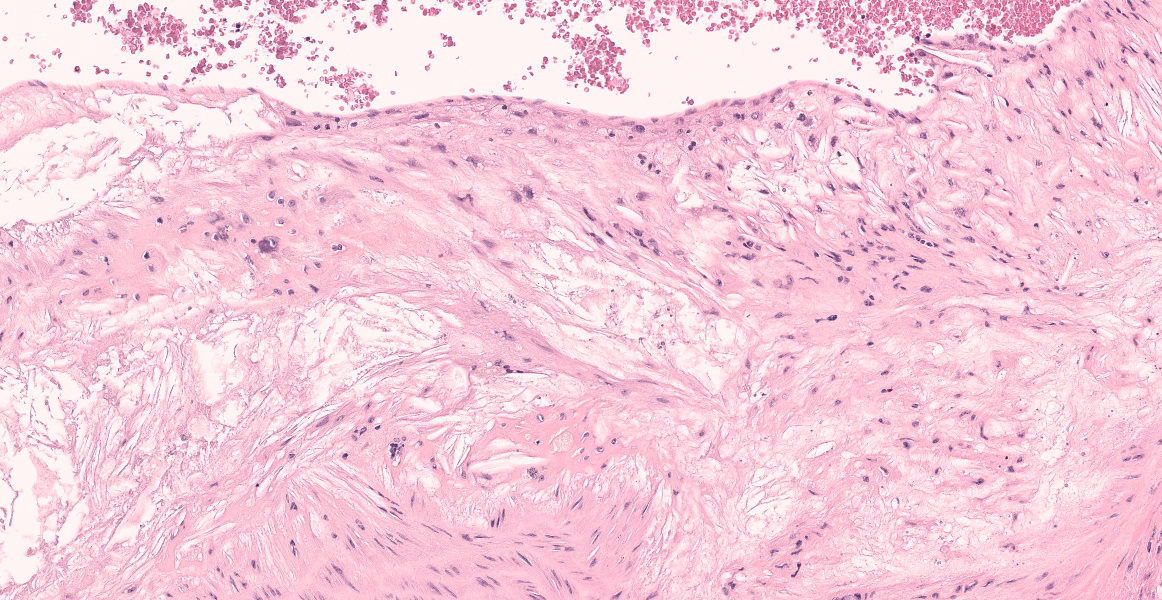
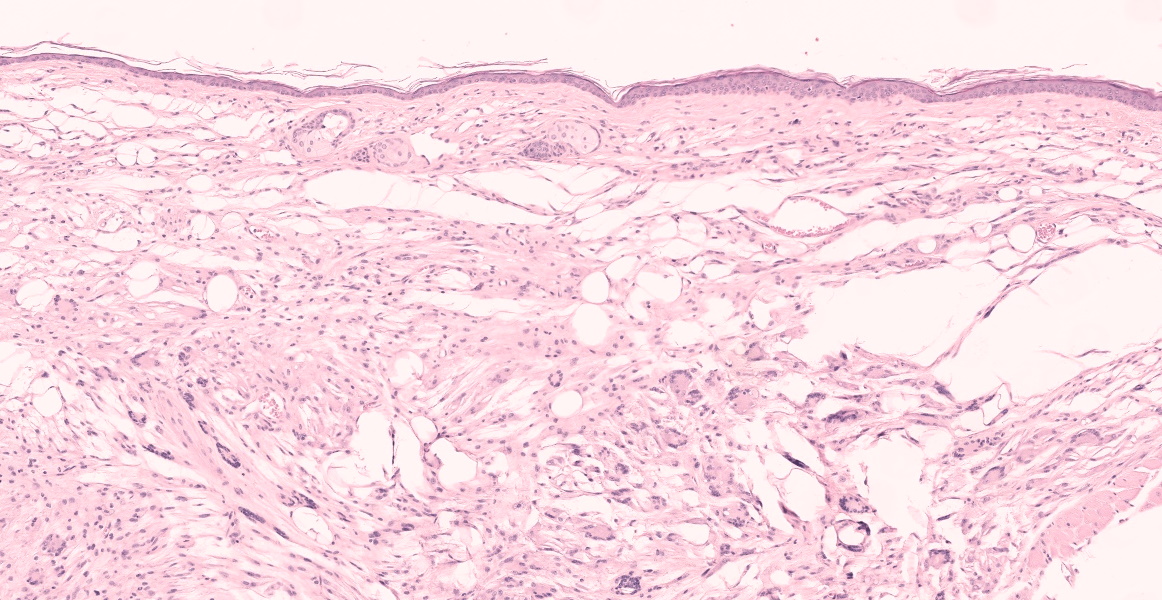
In multiple sections of skin, the epidermis is necrotic and ulcerated and the dermis is expanded by granulation tissue which supports abundant acicular cholesterol clefts, plump foamy macrophages, multinucleated giant cells, and fewer lymphocytes and plasma cells. Inflammation and cholesterol deposits extend from the superficial dermis into the deep muscular layers and subcutis. Inflammation occasionally encompasses nerve bundles and skeletal muscle myofibers are atrophied. Adjacent to the ulcers the epidermis is acanthotic and there are superficial serocellular crusts.
Contributor's Morphologic Diagnoses:
Heart (pulmonary artery, aorta, and vessels): Atherosclerosis, segmental, moderate.
Skin: Ulcerative dermatitis with dermal xanthomatosis, chronic, multifocal, severe.
Contributor's Comment:
Apolipoprotein E deficient (ApoE-/-) mice are useful for the study of mechanisms underlying cardiovascular disease and atherosclerosis, fat metabolism, and neurodegenerative diseases.3,8,16 ApoE is a structural component of lipoproteins that regulates lipid homeostasis and facilitates lipid transport. It functions as a ligand in the transport of many lipid products, such as cholesterol and fat-soluble vitamins, between cells and tissue.
Hypercholesterolemia is the primary clinical pathology finding characteristic of ApoE-/- mice.20 Subsequently, atherosclerosis and multicentric xanthomas (lipid mass-like accumulations) are well-recognized phenotypes of this model. Indeed, ApoE-/- mice can develop pre-atherosclerotic lesions, such as subintimal fatty streaks, in the proximal aorta as early as 3-months of age.16 Of interest to the current case, chronic hypercholesterolemia can alter epidermal lipid composition which may impair innate immune defense barriers of the skin which is associated with foam cell deposition in the dermis in both humans and in mice.1,9 Further, ApoE-/- mice fed a hypercholesterolemic diet exhibited xanthomatosis in many organs with a predilection for the subcutaneous and peritendinous tissues, similar to the skin lesions presented in this case. However, the ulcerative epidermal component in this mouse is somewhat unique.18 Given the C57/BL6 background idiopathic ulcerative dermatitis (UD), a commonly reported entity associated with pruritis in aging C57/BL6 mice, was considered as a top differential clinically. Indeed, wild type C57/BL6 mice fed a high-fat diet had worsened UD lesions compared to mice on standard rodent chow.5 In this case, it is suspected the implementation of a high fat diet exacerbated the lipogranulomatous dermal lesion and the presence of inflammation around nerve bundles may have contributed to the pruritis and subsequent ulcerative dermatitis noted clinically; however, initial UD may have also played a primary role. In conclusion, the clinical and histopathologic features of both ulcerative dermatitis and dermal xanthomatosis were considered in this case. The underlying etiologies of the skin lesion are thought to be driven by the genotype and further potentiated by both the high fat diet and UD.
Contributing Institution:
In Vivo Animal Core, University of Michigan, Unit for Laboratory Animal Medicine
https://animalcare.umich.edu/business-services/vivo-animal-core
ulam-ivac@umich.edu
JPC Diagnosis:
- Heart, arteries: Atherosclerosis, multifocal, marked.
- Heart, left ventricle: Fibrosis, subendocardial, multifocal, mild to moderate, with cardiomyocyte degeneration and loss.
- Ear pinna: Dermatitis, xanthogranulomatous, diffuse, marked with multifocal epidermal ulceration.
JPC Comment:
The term xanthoma is derived from xanthos, the Greek word for yellow.19 Xanthomas have been reported in numerous veterinary species and are relatively common in birds, less common in cats, and rare in dogs, horses, amphibians, and reptiles.13,14,15,17,19 Of the avian species, older psittacines are the most commonly affected, and lesions may occur in the skin of the abdomen, wings, and eyelids, with rare involvement of internal organs such as the bone marrow, liver, and ventriculus.15,17 In birds, dermal xanothomas are variably inflamed, often pruritic nodular masses that lead to self-mutilation. Chronic irritation, trauma, and high fat/high cholesterol diets are associated with xanthoma development in birds. In mammals, xanthomas may also be caused by high lipid diets, defective lipid metabolism, or underlying endocrine dysfunction (such as diabetes mellitus, hypothyroidism, hypoadrenocorticism, and pituitary pars intermedia dysfunction in horses).19 In dogs, a rare but striking form of xanthoma can occur in the eye secondary to the previously listed causes or lens-induced uveitis; grossly, affected eyes may be filled with yellow-tan material.7
Xanthomas in the central nervous system can lead to neurologic signs secondary to compression of adjacent parenchyma. In dogs, xanthogranulomas have rarely been reported in the sellar region. One recent report described xanthogranuloma intimately associated with a functional pituitary adenoma in a dog with hyperadrenocorticism and hypoparathyroidism; while the clinical signs in this case were attributed to the functional pituitary adenoma, another report described neurologic changes including aggression, seizures, and proprioceptive deficits due to compression by a xanthogranuloma in the pituitary region of a poodle with hyperadrenocorticism and hypothyroidism.2 A similar lesion, termed cholesteatoma, occurs in the choroid plexus of older horses secondary to recurrent mild hemorrhage.11 Large cholesteatomas obstruct the flow of cerebrospinal fluid causing secondary hydrocephalus.11
Buildup of lipid, lipid-laden macrophages, and cholesterol crystals in the tunica intima of large arteries, as demonstrated in this case, is termed atherosclerosis. Atherosclerosis is one of the most important diseases of humans and is a leading cause of mortality due to resulting myocardial infarction, cerebral infarction, aneurysm, and peripheral vascular disease. The complex pathogenesis involves a combination of hypercholesterolemia, endothelial dysfunction, and chronic inflammation.12 Intimal plaques occur in areas of turbulent blood flow and endothelial dysfunction and are initially composed of lipid-laden smooth muscle cells and macrophages. Extracellular oxidized LDL (generated by reactive oxygen species from previously activated macrophages) and phagocytosed cholesterol and fatty acids stimulate inflammasome formation, IL-1 production, and further activation of macrophages and T cells, perpetuating the inflammation. Cytokines and growth factors stimulate smooth muscle proliferation and collagen production, expanding the intimal plaque and creating a fibrous cap on the luminal surface. The plaque contains a core of lipid, lipid-laden cells, and possibly mineral, and if the fibrous cap is unstable and ruptures, this material is released into the vessel, causing thrombosis with vessel occlusion or embolization. Alternatively, outward pressure of the growing plaque can weaken the tunica media, resulting in aneurysm. Chronic atherosclerosis can also lead to gradual stenosis in smaller arteries, such as the coronary arteries, eventually resulting in tissue ischemia.
Clinical atherosclerosis in veterinary species is exceedingly rare, which creates a challenge when searching for animal models in this important human disease. Chickens, parrots, rabbits, pigs, and primates are susceptible to atherosclerosis, and while cats are generally considered atheroresistant, a recent report described clinical atherosclerosis in two related Korat breed cats with progressive cardiovascular failure.6 The histologic lesions were nearly identical to those seen in humans, and both had chronic myocarditis and fibrosis likely secondary to coronary artery stenosis and myocardial ischemia. In experimental models, pigs, rabbits, non-human primates, and mice are currently used, with the mouse being the most popular model in part due to its relative low cost and ease of genetic manipulation.4 ApoE or LDL deficient mouse models have provided tremendous insight into atherogenesis, but even they do not perfectly recapitulate the human disease, as they lack coronary stenosis and plaque instability.4
References:
- Bedja D, Yan W, Lad V, et al. Inhibition of glycosphingolipid synthesis reverses skin inflammation and hair loss in ApoE-/- mice fed western diet. Sci Rep. 2018;8(1).
- Fernandez-Gallego A, Del-Pozo J, Boag A, Maxwell S, Perez-Acino J. Xanthogranulomatous Pituitary Adenoma in a Dog with Typical Hyperadrenocorticism. J Comp Pathol. 2020;180: 115-121.
- Gao J, Katagiri H, Ishigaki Y, et al. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. 2007;56(1).
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32: 1104-1115.
- Hampton AL, Aslam MN, Naik MK, et al. Ulcerative dermatitis in C57BL/6NCrl mice on a low-fat or high-fat diet with or without a mineralized red-algae supplement. J Am Assoc Lab Anim Sci. 2015;54(5).
- Karkamo V, Airas N, Linden J, et al. Severe Spontaneous Atherosclerosis in Two Korat Breed Cats is Comparable to Human Atherosclerosis. J Comp Pathol. 2021;188: 52-61.
- Labelle P. The Eye. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th St. Louis, MO: Elsevier, Inc; 2021: 1429.
- Lewandowski CT, Maldonado Weng J, LaDu MJ. Alzheimer's disease pathology in APOE transgenic mouse models: The Who, What, When, Where, Why, and How. Neurobiol Dis. 2020;139. doi:10.1016/j.nbd.2020.104811
- Martins Cardoso R, Creemers E, Absalah S, et al. Hypercholesterolemia in young adult APOE -/- mice alters epidermal lipid composition and impairs barrier function. Biochim Biophys Acta - Mol Cell Biol Lipids. 2019;1864(7).
- Mitchell RN, Halushka MK. Blood Vessels. In: Kumar V, Abbas AK, Aster JC, eds. Robins & Cotran Pathologic Basis of Disease. 10th Philadelphia, PA: Elsevier, Inc; 2021: 493-504.
- Miller AD, Porter BF. Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th St. Louis, MO: Elsevier, Inc; 2021: 966.
- Oakes SA. Cell Injury, Cell Death, and Adaptations. In: Kumar V, Abbas AK, Aster JC, eds. Robins & Cotran Pathologic Basis of Disease. 10th ed. Philadelphia, PA: Elsevier, Inc; 2021: 62-63.
- Origgi FC. Lacertilia. In: Terio KA, McAloose D, St. Leger J, edgs. Pathology of Wildlife and Zoo Animals. Cambridge, MA: Elsevier, Inc: 2018: 879.
- Pessier AP. Amphibia. In: Terio KA, McAloose D, St. Leger J, edgs. Pathology of Wildlife and Zoo Animals. Cambridge, MA: Elsevier, Inc: 2018: 927.
- Reavill DR, Dorrestein G. Psittacines, Coliiformes, Musophagiformes, Cuculiformes. In: Terio KA, McAloose D, St. Leger J, edgs. Pathology of Wildlife and Zoo Animals. Cambridge, MA: Elsevier, Inc: 2018: 782.
- Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking Apo E - Evaluation of lesional development and progression. Arterioscler Thromb Vasc Biol. 1994;14(1).
- Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. 2nd Ames, IO: John Wiley & Sons, Inc; 2015. 73, 116, 194, 260, 264.
- van Ree JH, Gijbels MJJ, van den Broek WJAA, Hofker MH, Havekes LM. Atypical xanthomatosis in apolipoprotein E-deficient mice after cholesterol feeding. Atherosclerosis. 1995;112(2).
- Welle MM, Linder KE. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th St. Louis, MO: Elsevier, Inc; 2021: 1190, 1219.
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science (80- ). 1992;258(5081).