Signalment:
Gross Description:
Histopathologic Description:
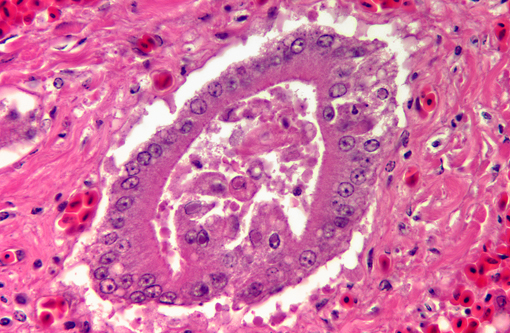
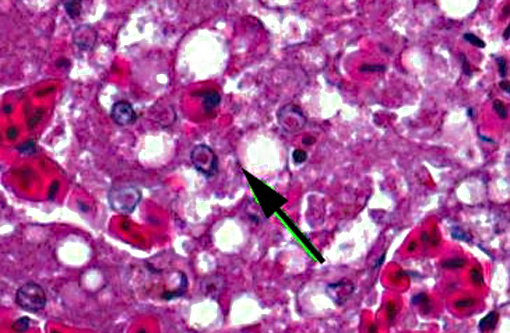
Liver: There is extensive disruption of hepatic architecture with moderate to large numbers of free erythrocytes (hemorrhage) interrupting hepatic cords. Individualized hepatocytes with eosinophilic and shrunken cytoplasm, and karyorrhectic or karyolytic nuclei are scattered throughout the section (individual cell necrosis). Additionally, there are multifocal, random areas of necrosis characterized by diffuse eosinophilia and variable retention of architecture. Hepatocytes in less affected portions of the liver frequently contain large, clear cytoplasmic vacuoles or have a lacy cytoplasmic appearance. Throughout the liver, in areas of necrosis, hemorrhage and architectural disruption, as well as in less affected areas, moderate numbers of hepatocytes contain eosinophilic intranuclear inclusion bodies. These are often centrally located with peripheral chromatin clearing. They occasionally fill the nucleus, leaving only a thin clear halo within the nuclear envelope. Similar intranuclear inclusion bodies are rarely found within biliary epithelial cells and affected cells are often sloughed into the ductular lumen. Necrotizing vasculitis, predominantly affecting central veins, is uncommon. There are multifocal, small, dense accumulations of bacteria (predominantly short bacilli) in sinusoidal and intravascular spaces and, occasionally, in foci of hepatocellular necrosis.
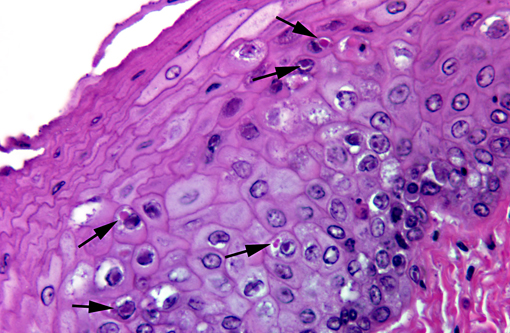
Esophagus: The mucosal epithelium contains multifocal ulcers and erosions and exhibits scattered necrosis of epithelial cells. There is disruption of normal layering, accumulation of karyorrhectic debris, few transmigrating leukocytes and variable intracellular edema (ballooning degeneration) in affected areas. Epithelial cells in these areas also occasionally contain intranuclear inclusion bodies similar to those described in the liver. Rare, variably sized, eosinophilic intracytoplasmic inclusion bodies are also present. Rafts of sloughed epithelial cells (with occasional intranuclear inclusion bodies) admixed with keratin, necrotic cell debris and mixed bacteria often cover the affected mucosa. Underlying some epithelial lesions is accumulation of karyorrhectic debris in the submucosa, accompanied by mild basophilia and disruption of the fibrillar collagen architecture. Submucosal glands are frequently obliterated; these glands contain intraluminal sloughed epithelia (with occasional intranuclear inclusion bodies), necrotic cellular debris and mucinous material. Intranuclear inclusion bodies are also present in remaining, viable glandular epithelium. Scant mononuclear infiltrates are scattered throughout the submucosa and are predominantly perivascular.
Morphologic Diagnosis:
1. Liver: Necrosis, acute, random, multifocal and individual cell, marked, with intranuclear inclusion bodies, necrotizing vasculitis, hemorrhage and hepatocellular vacuolar change.
2. Liver: Bacteremia, acute, moderate.
3. Esophagus: Necrosis, acute, multifocal, moderate, with intranuclear and intracytoplasmic inclusion bodies, multifocal erosions and ulcers, and submucosal gland necrosis.
Lab Results:
A Gram stain performed on the liver identified intravascular and intrasinusoidal Gram negative bacilli.
Condition:
Contributor Comment:
Based on the presence of intranuclear inclusion bodies, tissue necrosis and the species affected, anatid herpesvirus 1 (AnHV-1) was suspected and molecular diagnostics were pursued. PCR performed on a sample of frozen liver amplified an appropriately sized PCR product which was sequenced and found to be identical to AnHV-1 (DNA polymerase gene).
Anatid herpesvirus 1 causes predominantly a gastrointestinal disease in waterfowl which is referred to as duck plague or duck virus/viral enteritis (DVE).(1,3,4) Disease severity depends on the strain of the virus and species infected.(2) Muscovy ducks, like the one in this case, are considered to be one of the more sensitive species, while mallard ducks (Anas platyrhynchos) are more resistant.(3) Many anseriform species, including geese and swan, are susceptible, and recently a crested and a common coot (Fulica cristata and F. atria, respectively) reportedly died as a result of DVE.(4) As with other herpes viruses, ducks surviving initial infection become latently infected and can intermittently shed the virus.(3,4)
Typical presenting signs for DVE include lethargy, polydipsia, emesis, bloody or watery diarrhea and prolapse of the phallus.(3,4) In many cases, especially in the more sensitive species, there may be no clinical signs prior to the animal being found dead.(3,4) Infection typically occurs via oral ingestion of the virus, which is shed in the feces and orally from infected ducks; ingestion of contaminated water is thought to be the major route of transmission.(3,4) Typical gross lesions include petechiae in multiple organs, annular band necrosis and hemorrhage in the intestinal tract, ulcers and erosions in the esophagus (especially at the esophageal/proventriculus junction) and on the ventral surface of the tongue, and multifocal necrosis in the liver.(3,4) In addition to these findings, necrosis of lymphoid tissue and necrotizing vasculitis can be identified histologically.(1,3,4) Intranuclear inclusion bodies would be expected in areas of necrosis, including lymphocytes. Barr, et al(1) reported the presence of intracytoplasmic inclusion bodies in the esophagus and cloacal epithelium of DVE-affected Muscovy ducks.(1) Ultrastructurally, these inclusions were membrane-bound and contained enveloped visions.(1) Rare (and not present in all slides), variably sized eosinophilic intracytoplasmic inclusion bodies similar to those described in the above report were identified in the esophagus of this patient. Although other causes of intracytoplasmic inclusion bodies were not completely ruled out, they were considered less likely given the nature of the lesions, the findings in other organs and PCR identification of AnHV-1.
Approximately one week following the death of this duck, another Muscovy duck at the same facility was acutely lethargic one evening and found dead the following morning. Gross and histopathological lesions were similar to those reported here. This was the second DVE-related mortality event affecting ducks at our institutions in the past decade. The first was responsible for the death of four Muscovy ducks and at least two wild mallards. It is presumed that wild, latently infected ducks sharing waterways and housing with the Muscovies were responsible for the infection, but the possibility of latently infected permanent residents intermittently shedding the virus has not been fully investigated.
JPC Diagnosis:
1. Liver: Hepatitis, necrotizing, multifocal, moderate, with intranuclear viral inclusion bodies and necrotizing vasculitis.
2. Esophagus: Esophagitis, necrotizing, multifocal, moderate, with intranuclear and intracytoplasmic viral inclusion bodies and submucosal gland necrosis.
Conference Comment:
References:
2. MacLachlan NJ, Dubovi EJ. Herpesvirales. In: Fenners Veterinary Virology. 4th ed. London, UK: Acadmic Press Elsevier; 2011:179-201.
3. Ritchie BW, Carter K. Avian Viruses Function and Control. Lake Worth, FL: Wingers Publishing, Inc; 1995:207-214.
4. Salguero FJ, Sanchez-Cordon PJ, Nunez A, Gomez-Villamandos JC. Histopathological and ultrastructural changes associated with herpesvirus infection in waterfowl. Avian Pathol. 2002;31:133-140.