Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 17
5 February, 2020
CASE IV: AFIP 2018 053/18 (JPC 4119040).
Signalment: 5 year, 8-month-old spayed female DSH cat (Felis silvestris)
History: This cat presented with polyuria, polydipsia and cystitis. A diagnosis of diabetes mellitus was confirmed. The cat was treated with insulin, but blood glucose concentrations remained high despite treatment. For this reason, a presumptive diagnosis of insulin resistant diabetes was made, and the concentration of insulin-like growth factor (IGF)-1 in serum was measured. Later, the cat developed severe and recurrent vomiting, whereby it was humanely euthanized and submitted for necropsy.
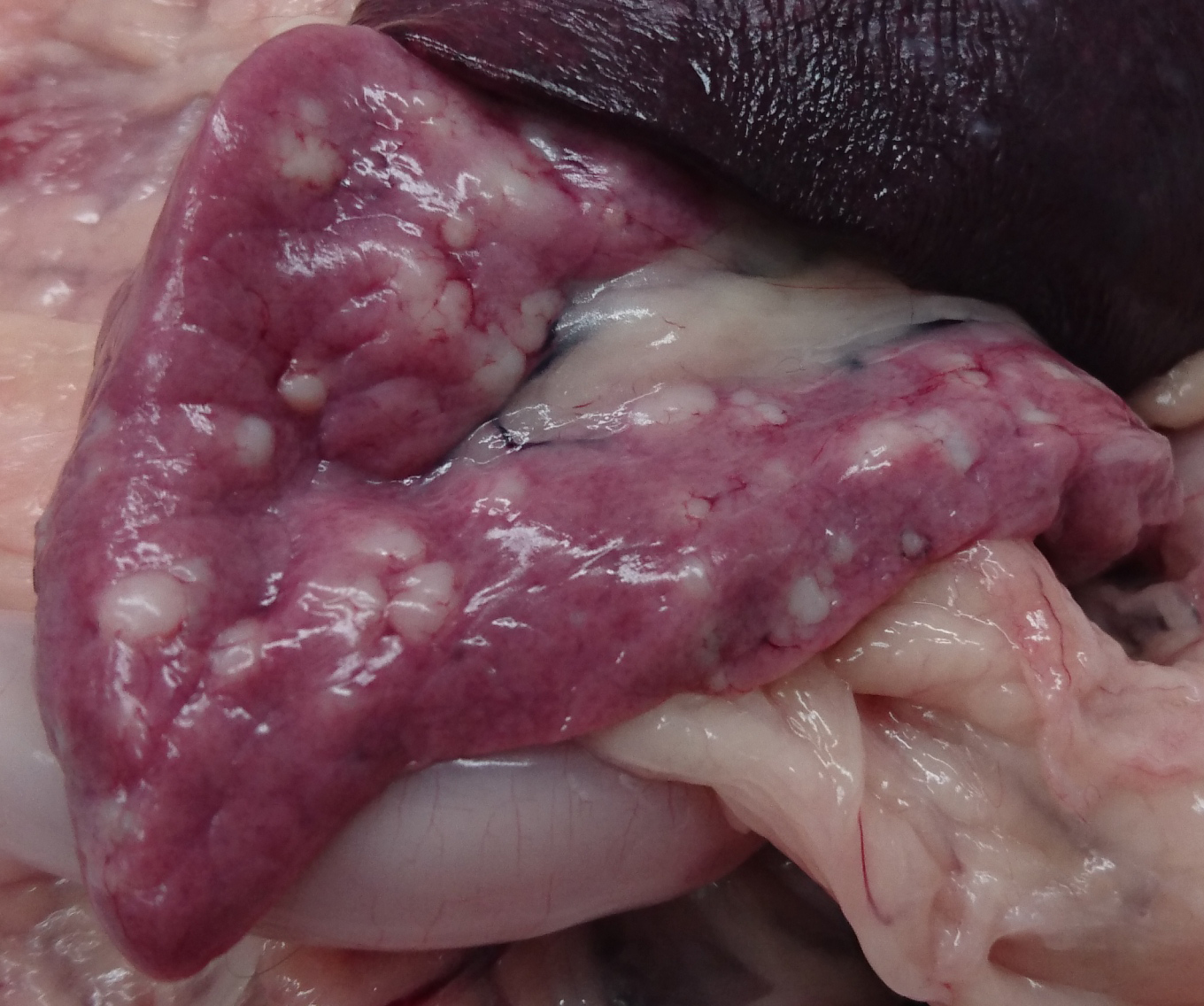
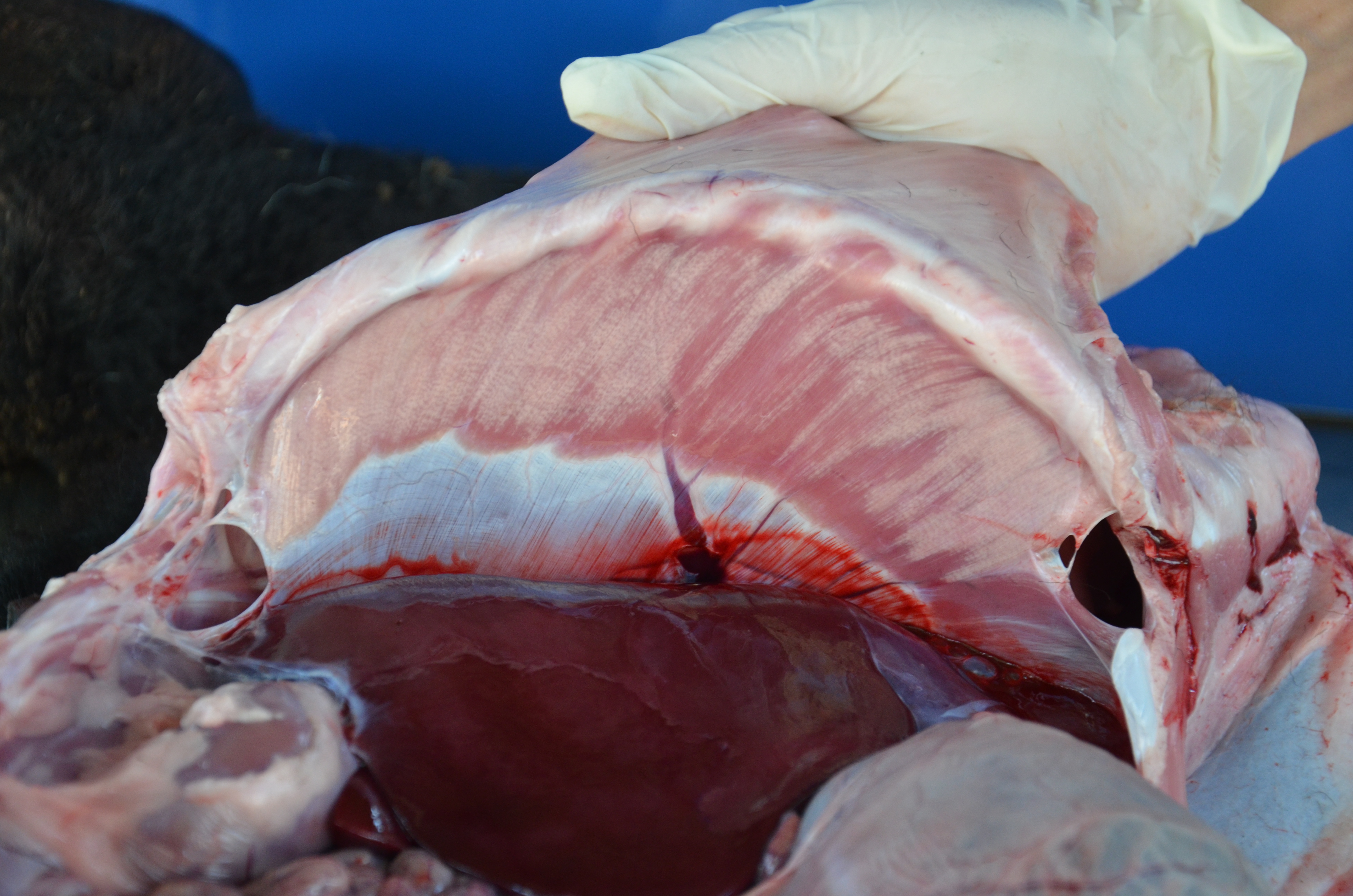
Gross Pathology: The cat was in good body condition with well-developed musculature. The pancreas was moderately enlarged and showed multiple lightly colored, homogenous, nodular structures measuring up to 5 mm in diameter. The pituitary gland was moderately enlarged.
Laboratory results: S-Glucose >20 mmol/l (reference range 3.5-6 mmol/l)
S-Fructosamine >500 µmol/l (reference range 190-350 µmol/l)
Serum Insulin-like Growth Factor (S-IGF-1) >3050 µg/l
(reference range 100-1200 µg/l)
Histologically, a well-circumscribed proliferation of well-differentiated
acidophil cells was confirmed in the pituitary gland, consistent with an
acidophil cell adenoma.
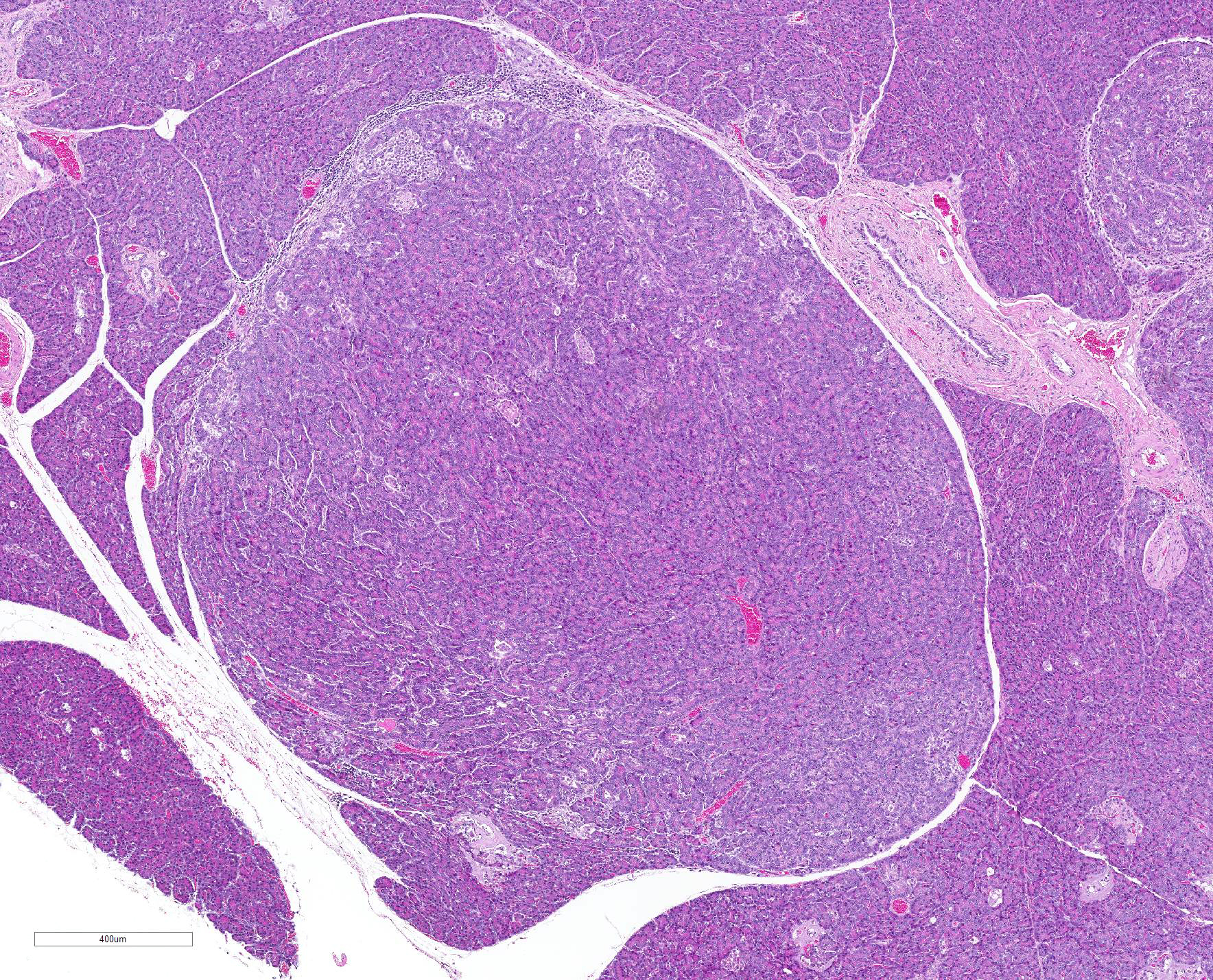
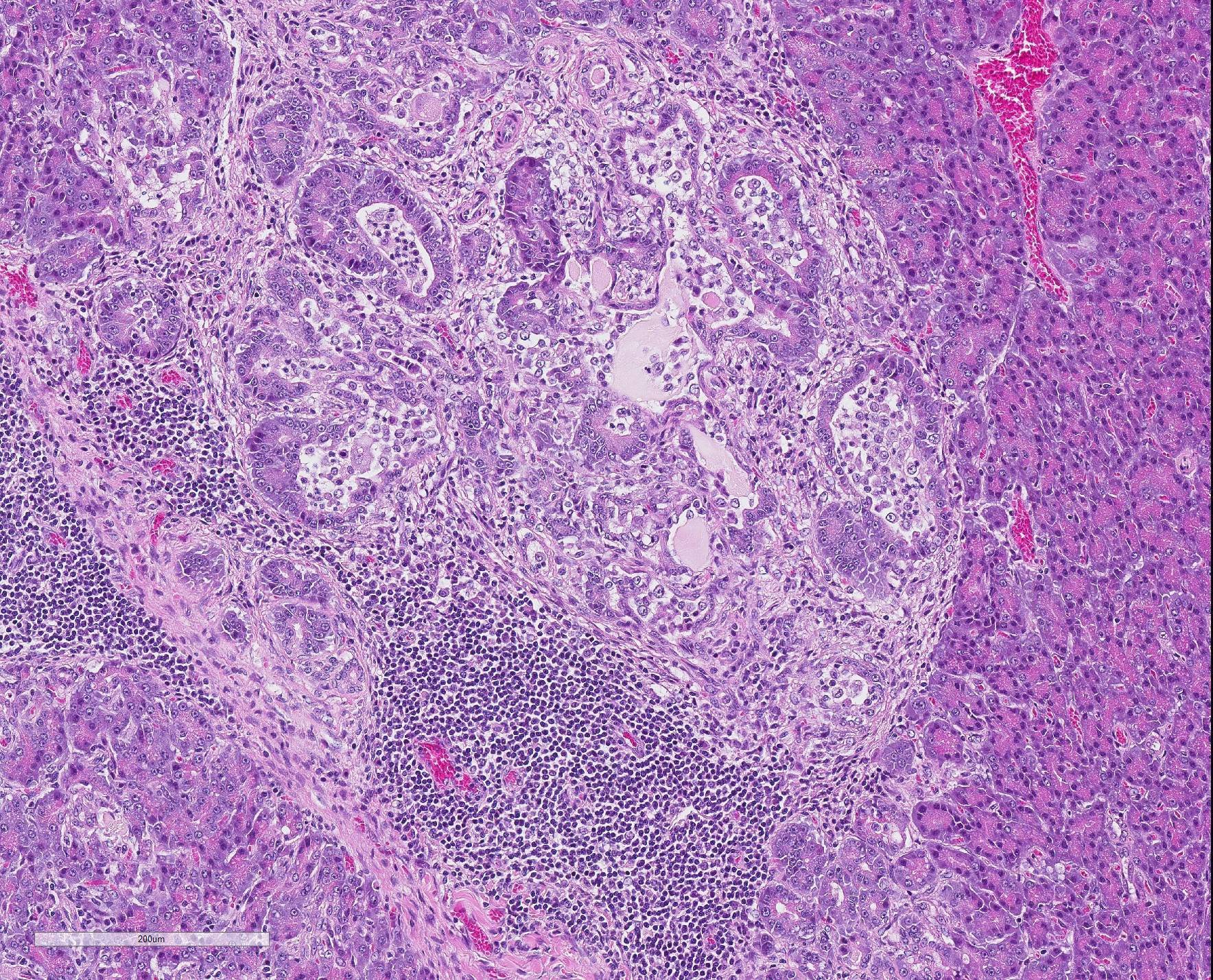
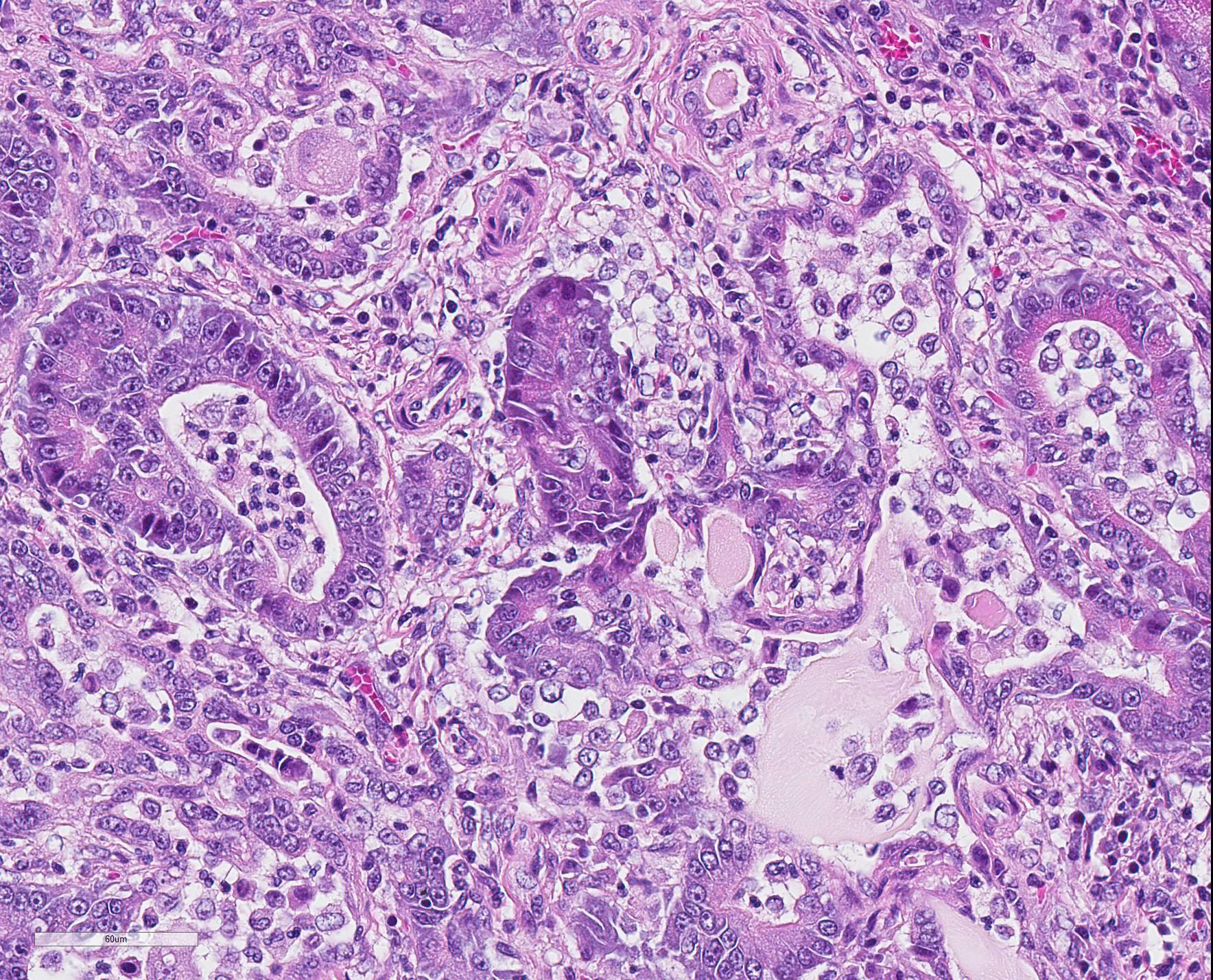
Microscopic Description: The exocrine parenchyma
shows several well-circumscribed nodules, composed of variable amounts of
well-differentiated acinar and ductal cells, often with reduced cytoplasmic
staining properties. Surrounding nodules, and multifocally separating lobules
as well as acinar and ductular structures, are variable amounts of fibrous
tissue, multifocally expanded by numerous lymphocytes and small numbers of
plasma cells. Multifocally, ductular structures are dilated and show epithelial
attenuation and loss. Within lumina are moderate numbers of neutrophils and
macrophages, as well as smaller amounts of eosinophilic, amorphous material
(secretion), occasionally admixed with cellular debris. Diffusely,
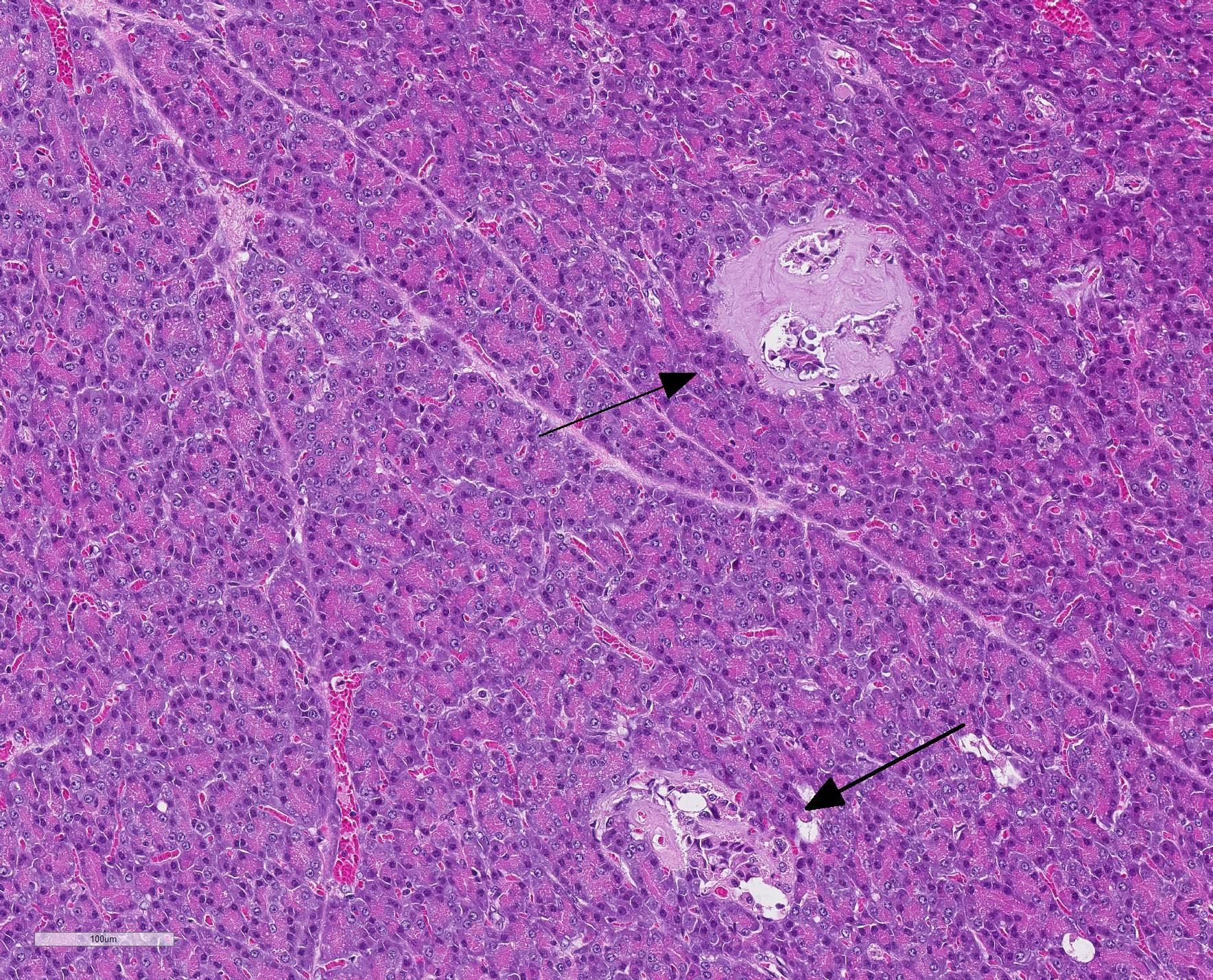
Langerhans?s islets show moderate to marked cellular loss and are expanded by
abundant amphophilic to lightly eosinophilic extracellular amorphous material
(amyloid). Multifocally, small numbers of cells within the islets show an
expanded vacuolated cytoplasm.
In Congo-red stained section (not provided), the extracellular material in the islets was confirmed to be amyloid by exhibiting apple-green birefringence in polarized light.
Contributor Morphologic Diagnosis:
1. Pancreas, multifocal nodular hyperplasia/adenomas
2. Pancreas, pancreatitis, multifocal, interstitial, chronic, moderate, lymphocytic with multifocal acinar/ductular dilation and neutrophilic inflammation
3. Pancreas: islet amyloidosis, diffuse, severe, with cellular degeneration and loss
Contributor Comment Diabetes mellitus is a disease characterized by loss of glycemic control with resulting fasting hyperglycemia. In cats, insulin resistant diabetes mellitus, resulting from inadequate tissue response to high insulin level is frequent, similar to diabetes mellitus type II in humans.10
Several pancreatic changes of the endocrine islets are associated with diabetes mellitus in cats, including loss of insulin-producing β-cells and amyloidosis of the islets. According to a recently published article, diabetic cats had approximately 65% lower median number of insulin-producing cells compared to control cats.18 The mechanism of amyloidosis is related to co-secretion of insulin and islet amyloid polypeptide (IAPP).11 The presence of amyloid can be verified histologically by staining with Congo Red, as was performed in this case, with amyloid protein exhibiting apple-green birefringence in polarized light.4 Thioflavin-S or T can also be used to detect amyloid, and may be useful due to poor staining with Congo red in cats.4,8 Historically, amyloidosis has been considered a consistent feature of diabetes mellitus in cats, and sometimes the cause of insulin dysregulation.9,16 However, this association and causality has been questioned in recent years, and a newly published paper failed to find increased deposits of amyloid in the pancreas of diabetic cats compared to controls.18
The degree to which inflammation plays a role in feline diabetes remains controversial. According to one study, the number of neutrophils and macrophages in pancreatic islets was similar between cats with diabetes mellitus and control cats, however the presence of T- and B-cells combined seemed more frequent in islets of diabetic cats compared to non-diabetic cats.18 In another study, expression of mediators regulating inflammation and oxidative stress was seen in cats with hyperglycemia and obesity, suggesting that these markers increase in the early development of diabetes mellitus.8
In addition to diabetes mellitus resulting from inadequate tissue response to high insulin level, a known cause of feline diabetes mellitus is acromegaly.16 Acromegaly is a clinical condition characterized by overgrowth of connective tissue and enlargement of viscera, resulting from chronic excessive secretion of growth hormone (GH), also known as hypersomatotropism.16,19 According to one author, nodular pancreatic hyperplasia is commonly encountered in acromegalic cats on post mortem examinations.14 Acromegaly may cause diabetes mellitus due to a GH-induced postreceptor defect in insulin action in target tissues.14 GH measurements in blood has traditionally been used to diagnose acromegaly. However, S-IGF1 has replaced this parameter in recent years, due to limited GH assay availability.3,13 Presence of an acidophil cell adenoma in the pituitary gland, together with severely elevated serum IGF values, suggest that the diabetes mellitus in this case was a result of hypersomatotropism due to neoplastic GH-secretion.
Pancreatitis is frequently diagnosed in cats,; the chronic form being more prevalent than the acute form.2 In the acute form, neutrophilic infiltrates along with acinar cell and peripancreatic fat necrosis is observed, meanwhile chronic pancreatitis is dominated by non-suppurative infiltrates of lymphocytes, accompanied by fibrosis and acinar atrophy.6,10 Pancreatitis in cats may coexist with inflammatory bowel disease and cholangitis.1 Moreover, pancreatitis is often observed in conjunction with a diagnosis of diabetes mellitus, and may be subclinical.5,18 Anecdotally, the concurrent condition of diabetes mellitus and pancreatitis can make glycemic control more ?brittle?.5 However, it is not clear whether the pancreatitis leads to diabetes mellitus, or if the diabetes mellitus causes pancreatitis.5 It has been shown that the number of neutrophils is increased in pancreatic exocrine tissue in cats with hyperglycemia.18 According to another study, the degree of inflammation of the exocrine pancreas is not significantly associated with diabetes nor ketoacidosis. However, the mitotic index of acinar cells, as measured by Ki67 and PCNA, is increased in cells in the near vicinity of pancreatic islets in cats with diabetes, possibly due to chronic pancreatitis.17
The gross nodular changes in the pancreas corresponded histologically to well-defined benign proliferations of exocrine pancreatic tissue. In summary, these were determined to be representative of nodular hyperplasias or adenomas. Nodular hyperplasia can be seen in several organ systems of aging individuals including the liver, adrenals, thyroid glands, spleen and pancreas.10,13,17 In the pancreas these changes are usually considered clinically silent. In other organs, nodular hyperplasia has been discussed as a response to tissue injury or due to chronic pituitary hormone stimulation.12 In this case, it is unclear if the nodular changes are purely hyperplasic or represent an adenomatous change. Possibly, the chronic inflammation could contribute to the increased amount of fibrous tissue surrounding nodules.
Contributing Institution:
Swedish University of Agricultural Sciences
Department of Biomedical Sciences and Veterinary Public Health, Section of Pathology
BOX 7028
SE 750 07, Uppsala, Sweden
https://www.slu.se/en/departments/biomedical-sciences-veterinary-public-health/
JPC Diagnosis: 1. Pancreas: Pancreatitis, interstitial, necrotizing and lymphohistiocytic, chronic, multifocal mild to moderate.
2. Pancreas, islets of Langerhans: Amyloidosis, diffuse severe.
3. Pancreas, exocrine tissue: Hyperplasia, multifocal, moderate.
JPC Comment: While the theoretical interplay of the three visualized lesions of chronic interstitial pancreatitis, islet amyloidosis, and exocrine hyperplasia, as well as the fourth lesion of an enlarged pituitary (which was neither submitted or further described in this case) and its discussion by the contributor is interesting to ponder; the participants had seen these three lesions independently and in various combination frequently in older cats, and were reticent to assign a relationship between any of the three, or the concomitant increased levels of serum growth hormone. (Also interesting is that this particular cat was less than 6 years of age.) Amylin, or islet associated peptide is toxic to islet cells in addition to resulting in effacement and atrophy as a result, but the presence of islet amyloid in the cat should not be interpreted as prima facie evidence of diabetes in the cat.10
The contributor mentions the possibility of elevated growth
hormone levels and acromegaly in this particular individual as a potential
cause of diabetes. Diabetes in acromegalic cats is a result of the catabolic
effects of growth hormone, which causes insulin resistance through the
generation of a postreceptor defect in the action of insulin or target cells
with resulting diminished carbohydrate utilization as well as gluconeogenesis.7
Various studies have demonstrated the concurrent presence of diabetes mellitus
in between 20 and 50% of acromegalic human patients. One study of 1221
diabetic cats demonstrated 26.1% to have elevated serum insulin-like growth
factor (secreted by the liver) and of this subset, 89% demonstrated pituitary
neoplasms.15
Another effect of growth hormone release by a pituitary somatotroph tumor in an
acromegalic patient is due to the anabolic effects of insulin-like growth
factors, which results in increased protein synthesis and tissue growth.
Affected cats demonstrate a characteristic clinical appearance (colloquially referred
to as a "gangster cat" due to increased body weight, prognathia inferior, a
broad face, enlarged paws, respiratory stridor, and often cardiac and abdominal
organomegaly). While not seen in all patients, these changes were not
documented in the history of this case.7
Chronic interstitial pancreatitis is the typical pattern of inflammation of the pancreas in species other than the dog, and in cats, generally arises from an inflammatory process beginning in the ducts. Histologically, as seen in this case, ducts may contain exudate, may be dilated or stenotic, and are often surrounded by bands of fibrous connective tissue which may communicate with the interlobular stroma. Aggregates of lymphocytes and plasma cells are not uncommon in inflamed areas.10
References:
1. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med. 2012;27(3):140-7.
2. Bazelle J, Watson P. Pancreatitis in cats: is it acute, is it chronic, is it significant? J Feline Med Surg. 2014;16(5):395-406.
3. Berg RI, Nelson RW, Feldman EC et al. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med. 2007;21(5):892-8.
4. Cianciolo, RE, Mohr, FC. Urinary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Philadelphia, PA:Saunders Elsevier; 2016:377-463.
5. Davison, J. Diabetes mellitus and pancreatitis--cause or effect? Small Anim Pract. 2015;56(1):50-9.
6. De Cock HEV, Forman MA, Farver TB et al. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol. 2007;44(1):39-49.
7. Fracassi F, Salsi M, Sammartano F, Bo, Kooistra HS. Acromegaly in a non-diabetic cat. J Fel Med Surg Open Rep 2(1):2055116916646585. doi: 10.1177/2055116916646585.
8. Herndon AM, Breshears MA, McFarlane D. Oxidative modification, inflammation and amyloid in the normal and diabetic cat pancreas. J Comp Pathol. 2014;151(4):352-62.
9. Johnson KH, O?Brien TD, Jordan K et al. Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989;135(2):245?250.
10. Jubb, KVF, Stent, AW. Pancreas. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Philadelphia, PA:Saunders Elsevier; 2016:353-373.
11. Kahn SE, D'Alessio DA, Schwartz MW et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes. 1990;39(5):634-8.
12. Newman SJ, Steiner JM, Woosley K et al. Correlation of age and incidence of pancreatic exocrine nodular hyperplasia in the dog. Vet Pathol. 2005;42(4):510-3.
13. Niessen SJ. Feline acromegaly: an essential differential diagnosis for the difficult diabetic. J Feline Med Surg. 2010;12(1):15-23.
14. Niessen SJ, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Anim Pract. 2013;43(2):319-50.
15. Niessen SJ, Forcada Y, Mantis P, Lamb CR, Harrington N, Fowkes R, Korbonis M, Smith K, Church. Studying Cat (Felis catus) diabetes: Beware of the acromegalic imposter. PLoS One 2015; 29;10(5):e0127794. doi: 10.1371/journal.pone.0127794.
16. O?Brien TD. Pathogenesis of feline diabetes mellitus. Mol Cell Endocrinol. 2002;29:197(1-2):213-9.
17. Rosol, TJ., Gröne, A. Endocrine Glands. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th ed. Philadelphia, PA:Saunders Elsevier; 2016:270-356.
18. Zini E, Osto M, Moretti S et al. Hyperglycaemia but not hyperlipidaemia decreases serum amylase and increases neutrophils in the exocrine pancreas of cats. Res Vet Sci. 2010;89(1):20-6.