Signalment:
Gross Description:
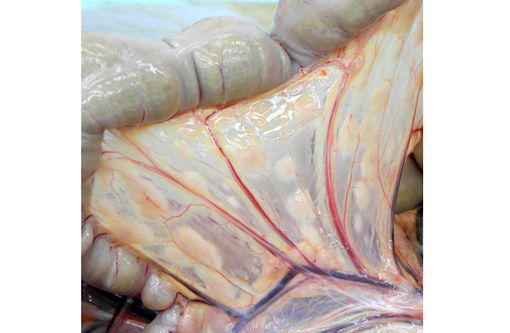
The mesenteric lymph node was severely enlarged and measured 5.0 x 3.5 x 2.0 cm, and was tan to white on cut surface. A focal tan-white lesion was present within the jejunum with prominent thickening of the wall over a length of 4.5 cm. The colonic and pancreatic lymph nodes were moderately enlarged. The spleen was mildly enlarged and there were multiple nodules within the parenchyma which were irregular, tan-white and up to 1.5 cm in diameter. The wall of the gallbladder was moderately to severely thickened and was white to tan. These lesions were consistent with lymphoma.Â
The heart, kidneys, stomach, duodenum, ileum, cecum, colon and testes appeared normal. Several adhesions were present between the right caudal lung lobe and thoracic wall. The lungs otherwise appeared grossly normal.
Histopathologic Description:
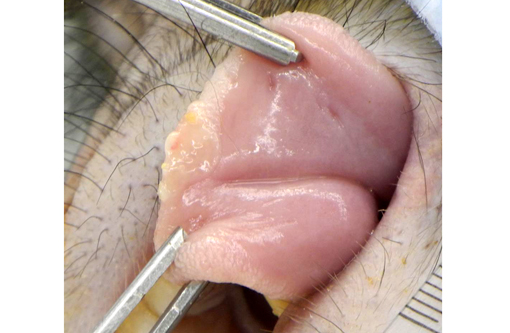
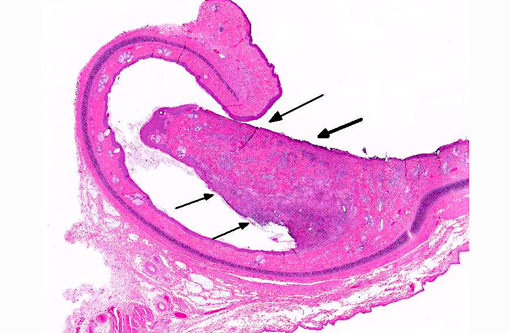
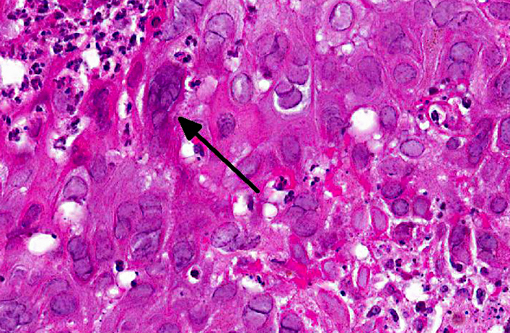
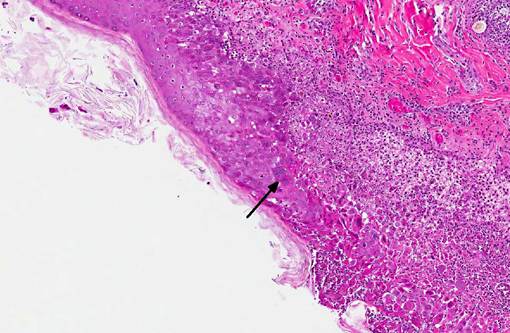
Examination of the tongue (not submitted) revealed an acute focal ulcerative glossitis with the margins of the lesion containing epithelial cells with prominent amphophilic intranuclear inclusion bodies. Transmission electron microscopy from a section of tongue revealed intranuclear icosahedral viral nucleocapsid particles measuring approximately 100 nm, and enveloped particles approximately 160 nm in diameter in the cytoplasm, consistent with herpesviral particles.Â
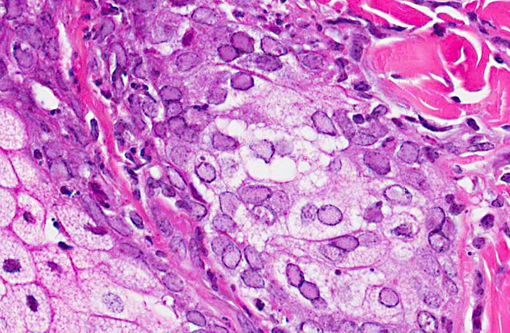
Other significant findings in this case included lymphoblastic lymphoma affecting the spleen, jejunum, gall bladder, adrenal gland, and mesenteric, perisplenic, pancreatic and colonic lymph nodes. Additionally, there was a granulomatous colitis and lymphadenitis affecting a portion of the colon and associated lymph nodes due to mycobacterial infection, with large numbers of acid fast bacilli evident in macrophages in the affected tissue. Mild to moderate multifocal interstitial pneumonia and fibrosis were evident in the lung.Â
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Macacine herpesvirus-1, previously known as Cercopithecine herpesvirus-1, Herpesvirus simiae and Herpes B virus is a common alpha herpesvirus infection affecting Old World macaques. It has been documented in rhesus macaques (Macaca mulatta), cynomolgus macaques (M. fascicularis), stumptail macaques (M. artoides), pigtailed macaques (M. nemestrina), Japanese macaques (M. fuscata), bonnet macaques (M. radiata) and Taiwan macaques (M. cyclopis). Natural infection in macaques is usually asymptomatic, with localized mucosal lesions occurring infrequently. Transmission is horizontal between macaques with most animals acquiring the infection by 2-4 years of age. Lesions when present typically involve the oral and gingival mucosa, tongue and conjunctiva. Genital lesions are generally not observed.(5) Infection of New World monkeys, while uncommon, is also possible. Asymptomatic natural infection with macacine herpesvirus-1 has been documented in a colony of brown capuchin monkeys (Cebus apella).(3)
Macacine herpesvirus-1 has typical morphology for alpha-herpesviruses and is composed of double stranded DNA, with a 40 nm core, icosahedral nucleocapsid approximately 100 nm in diameter and enveloped particles of 160-180 nm in the infected cell cytoplasm. Host cell infection occurs by fusion of viral envelope with host cell plasma membranes, penetration of nuclear pores by viral capsids with release of viral DNA in the nucleus, leading to viral replication.(6)
Transmission typically occurs via exposure of oral, ocular or genital mucous membranes from an infected animal shedding virus most commonly in saliva. The virus replicates locally, enters sensory nerves and is transmitted intra-axonally where it can remain latent in sensory ganglia, protected from host immune responses. Reactivation can occur periodically from the latent state, with virus being transported down axons to mucosal tissues. The majority of reactivated infections are asymptomatic. Virus carriers generally have a low rate of shedding, however, stress associated with transportation, changes in social groupings and immunosuppression can lead to reactivation and viral shedding.(4)
While generally not a significant clinical disease entity for immunocompetent macaques, cases of systemic infection have been documented in cynomolgus macaques causing necrosis of lung, liver, spleen, pancreas, and adrenal glands.(2,7) In one of these cases infection with Simian retrovirus type D was noted as the likely cause of immunosuppression leading to pathogenic herpesvirus infection.(2)
Macacine herpesvirus is an extremely important zoonotic agent. Human exposure to an infected macaque shedding virus can result in a highly pathogenic infection leading to CNS involvement and a fatality rate greater than 70% in individuals who are not treated aggressively with antiviral drugs.(1,4) Universal precautions with appropriate PPE are required when working with nonhuman primates. Any exposure by scratch, bite, needle stick injury or any other exposure of mucous membranes or an open skin wound require immediate attention and consultation with occupational medical specialists.Â
JPC Diagnosis:
Conference Comment:
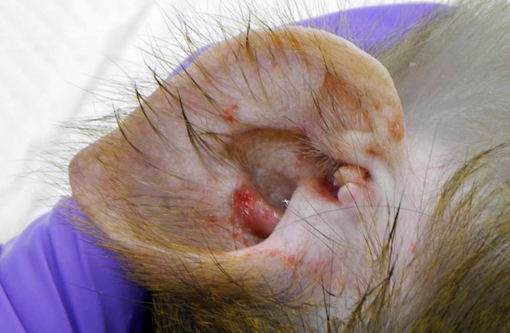
Overall, this rhesus macaque had lymphoma, α-herpesviral glossitis and otitis externa, granulomatous lymphadenitis/colitis (likely secondary to M. avium) and alveolitis, interstitial pneumonia and fibrosis.Â
References:
2. Carlson CS, OSullivan MG, Jayo MJ, et al. Fatal disseminated cercopithecine herpesvirus 1 (herpes B infection in cynomolgus monkeys (Macaca fascicularis). Vet. Pathol. 1997;34(5):405-14.
3. Coulibaly C, Hack R, Seidl J, et al. A natural asymptomatic herpes B virus infection in a colony of laboratory brown capuchin monkeys (Cebus apella). Lab. Anim. 2004;38(4):432-8.
4. Elmore D, Eberle R. Monkey B virus (Cercopithecine herpesvirus 1). Comp. Med. 2008;58(1):11-21.
5. Huff JL, Barry PA. B virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg. Infect. Dis. 2003;9(2): 246-50.
6. Jainkittivong A, Langlais RP. Herpes B virus infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998;85(4):399-403.
7. Simon MA, Daniel MD, Lee-Parritz D, et al. Disseminated B virus infection in a cynomolgus monkey. Lab. Anim. Sci. 1993;43(6):545-50.