CASE 4: 17030960 (4101142-00)
Signalment: Two ducks: A 1-year-old, intact female, Muscovy duck, Cairina moschata and a 1-year-old, intact male, Muscovy duck, Cairina moschata
History:
Two ducks presented to the Oklahoma Animal Disease Diagnostic Lab on March 16th, 2017 for autopsy. Over a period of five days, approximately 25 ducks, all of which were Muscovy ducks, had died at Bethany Pond in the owner's front yard. She had cared for these animals since birth and had pet ducks that were not being affected; therefore, she raised concerns of intoxication rather than infectious agents. A similar die off had occurred previously in June of 2016 when approximately 24 ducks were found dead.
Gross Pathology:
Postmortem examination was performed on an adult female duck. The animal was in good body condition based on normal skeletal muscle mass and adequate adipose tissue stores. All tissues were mildly to moderately autolytic. No gross lesions were found.
Postmortem examination also found the adult male duck to be in good body condition with mild to moderate autolysis. The liver contained three, multifocal, discrete, 1mm diameter, firm, dark green foci on the capsular surface. No other gross lesions were observed.
Laboratory results:
All laboratory testing was completed at the National Wildlife Health Center in Madison, WI.
- Results of RT-PCR for Duck Virus Enteritis (anatid herpesvirus - 1) performed on liver tissue were positive.
- Virus isolation for Duck Virus Enteritis (anatid herpesvirus - 1) performed on liver specimens was negative.
- Results of RT-PCR for Avian Influenza virus performed on trachea (routine screening on avian necropsy specimens) were negative.
Microscopic description:
Microscopic lesions are similar in both animals and are described together.
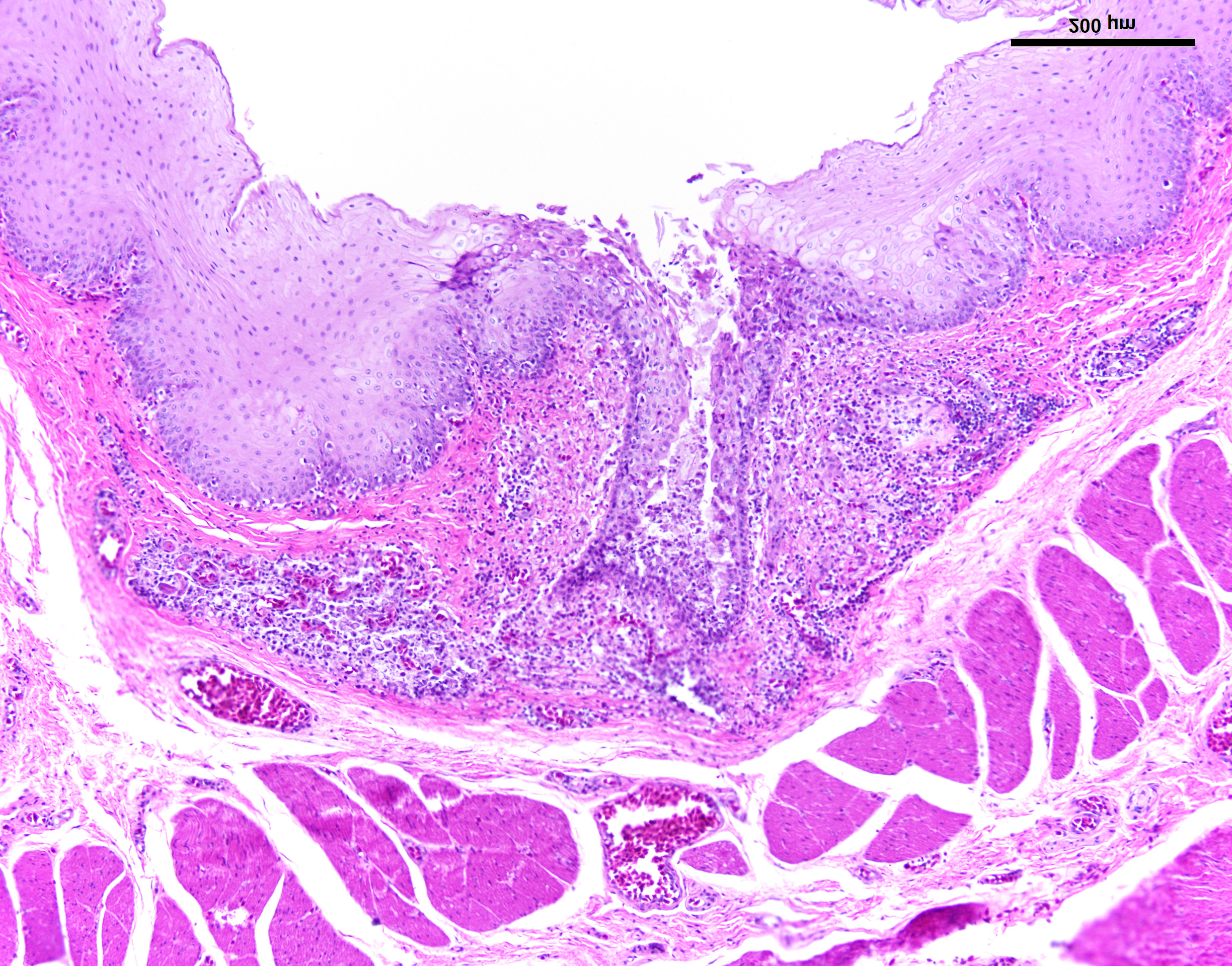
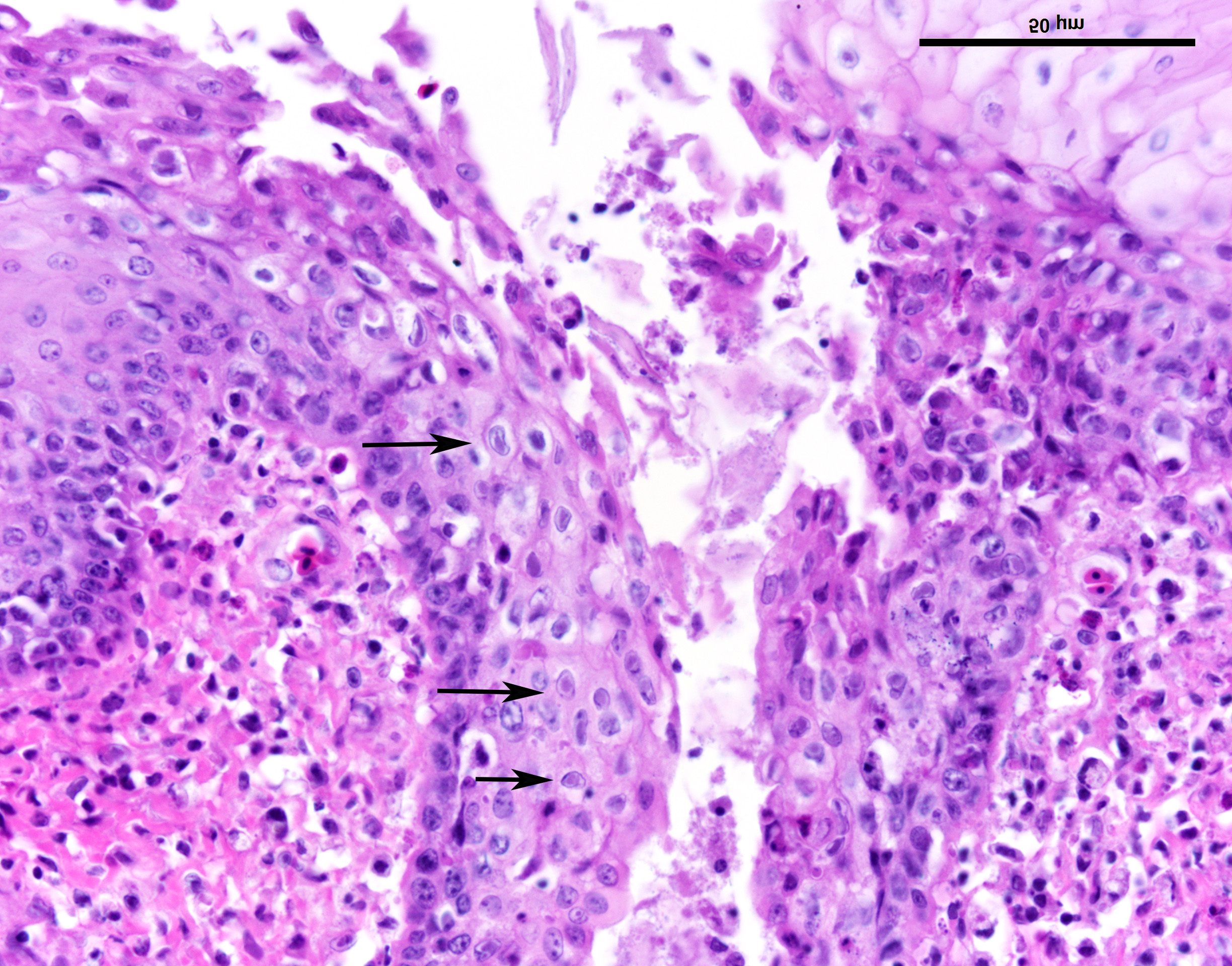
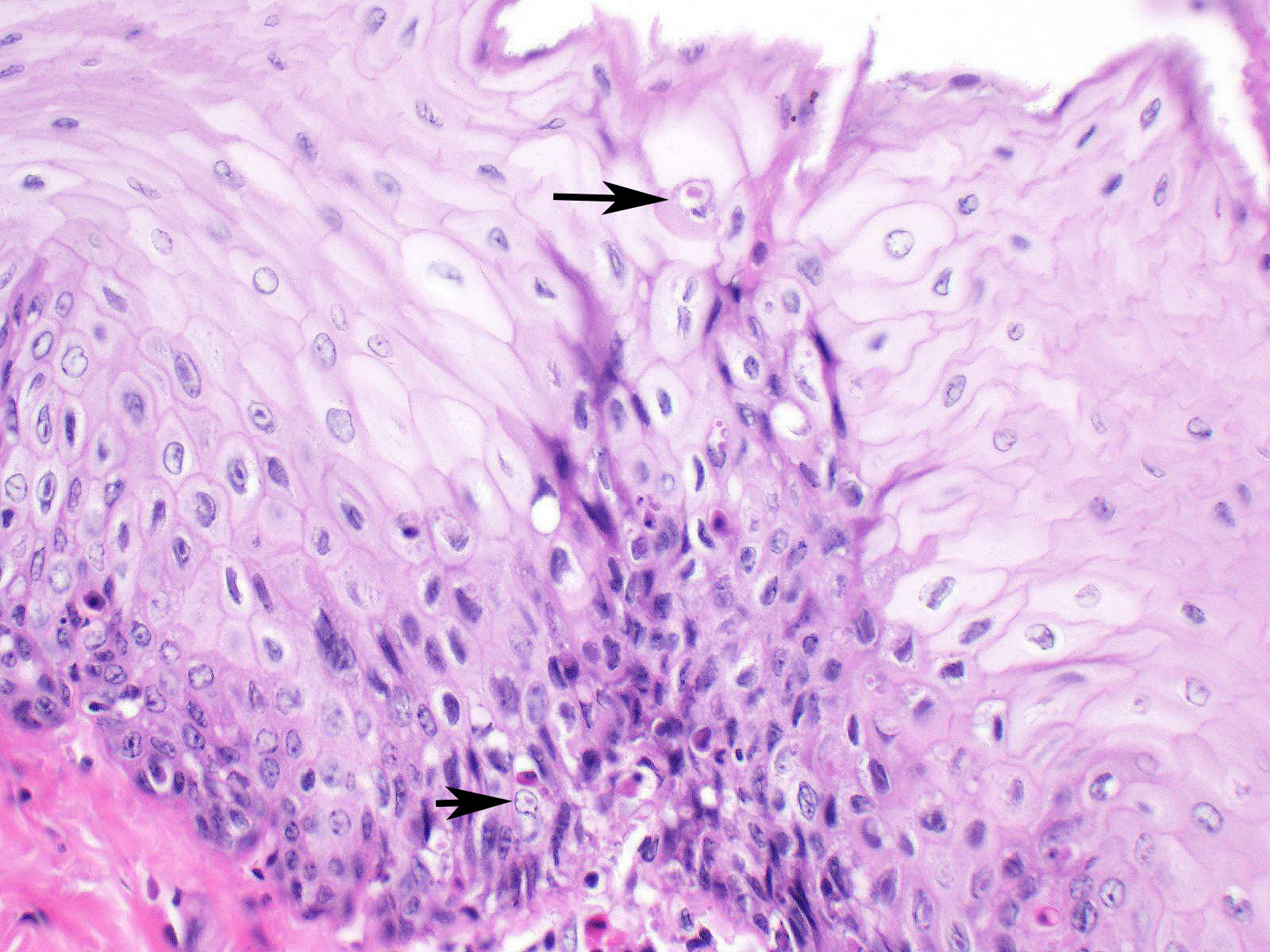
Esophagus: Multifocally, lumens of submucosal glands contain sloughed, necrotic epithelial cells admixed with apoptotic and necrotic macrophages and lymphocytes. Adjacent lymphoid aggregates also contain necrotic lymphocytes and cellular debris. Epithelium overlying the affected glands and lymphoid aggregates have ballooning degeneration in the stratum spinosum that progresses to necrosis with mucosal erosion or ulceration and accompanying inflammation. These areas of affected epithelium are typically continuous with the underlying gland. Large numbers of mucosal epithelial cells adjacent to these areas contain single 1 µm x 3 µm diameter, smudgy, magenta, intranuclear inclusion bodies that displace chromatin to the nuclear periphery, forming a halo.
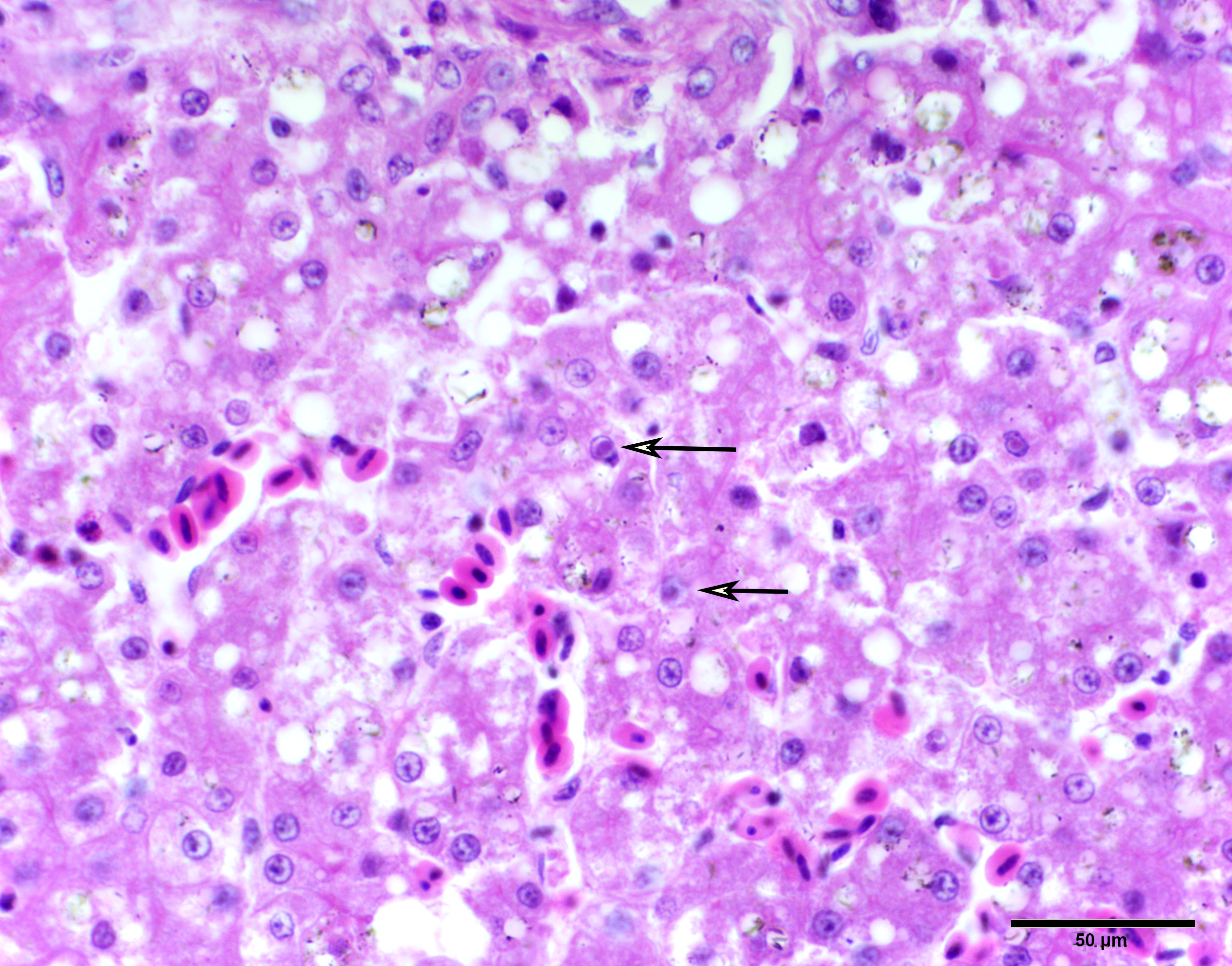
Liver: Randomly scattered to occasionally coalescing foci of hepatocellular necrosis are surrounded by hepatocytes that contain intranuclear inclusion bodies similar to those previously described. In areas not interrupted by hepatocellular necrosis, individual scattered hepatocytes have intranuclear inclusion bodies. Throughout the liver, hepatocytes have periportal to diffuse microvesicular and macrovesicular cytoplasmic vacuolation. Sinusoids are mildly ectatic and congested.
Proventriculus: Previously described intranuclear inclusions are within epithelial cells scattered throughout the section without additional lesions that are consistent on all slides.
Contributor's morphologic diagnosis:
Esophagus: Moderate to marked, acute, multifocal, erosive and ulcerative esophagitis with ballooning degeneration and submucosal glandular necrosis with intranuclear inclusion bodies consistent with anatid herpesvirus - 1
Liver: Moderate, acute, multifocal hepatocellular necrosis with intranuclear inclusion bodies consistent with anatid herpesvirus - 1 and periportal to diffuse hepatocellular vacuolation
Contributor's comment:
Duck Virus Enteritis (DVE), also known as Duck Plague is caused by anatid herpesvirus-1, a member of the alpha herpesviradae subfamily.2 This infection affects a wide range of birds in the Anseriformes order with varying disease severity depending on viral pathogenicity, and age and species of the animals.1,5 Mallard ducks along with Pintail ducks are considered to be the least susceptible to DVE and are implicated as carriers whereas Blue Winged Teal ducks and Muscovy ducks are highly susceptible to fatal infection.1, 2,7 Young ducklings aged 2-7 weeks have been shown to be less severely affected in comparison to adult ducks.7 DVE was first observed in 1923 and later described and distinguished from avian influenza in 1942.1 Outbreaks have occurred worldwide, are seasonal, and tend occur near bodies of water. Clinical signs are variable and often nonspecific including sudden death in birds with good body condition, a drop in egg production, anorexia, depression, ruffled feathers, diarrhea, hematochezia, penile prolapse, nasal discharge, epiphora, photophobia, and periocular crusting.1,5,6 Carrier birds can have sublingual ulcers.6 Gross lesions typically include annular bands of necrotic intestinal mucosa with overlying bands of hemorrhage and pseudo membrane formation, hemorrhage into body cavities and/or into the intestinal lumen, and myocardial, hepatic, and ovarian hemorrhage with necrosis.1,5,6 Microscopically, the gastrointestinal tract is primarily affected along with lymphoid organs; however, this virus is considered pantropic with lesions and intranuclear or intracytoplasmic viral inclusions found in multiple tissues.2,5 DVE is transmitted horizontally from infected animals by either direct contact or indirectly through environmental contamination. Vertical transmission has only been observed experimentally.1,2 Recovered birds are immune to reinfection; however, they become carriers with latent infections in the trigeminal ganglion and shed virus in times of stress, silently adding to environmental contamination.5 Cell culture is the gold standard in diagnostics for DVE, although PCR and serology in combination with electron microscopy are also useful diagnostic tools.5 There is no effective treatment for DVE and control relies heavily on management, depopulation, and communication with state and federal agencies.1 Live attenuated and killed vaccines are available for production animals but have not been distributed to the general public.
The outbreak within this flock spared Mallard ducks and killed all Muscovy ducks, which is consistent with the literature. Ducks in our case were also lacking gross lesions, which supports the sensitivity of this species to DVE. In 1987, Wobeser described a similar lack of gross lesions in Blue-Winged Teal ducks. Although the paper speculated that may have been due to a more pathogenic strain of virus, we hypothesize that the lack of gross lesions in these two species may be due to the more rapid disease course and death found in highly susceptible animals. This is also demonstrated in the multifocal distribution of the esophageal lesions in our ducks. The literature describes diffuse lesions in most species that have longer average times to death. Wobeser also documented microscopic lesions within the Blue-winged Teal ducks that mirror lesions found in our Muscovy ducks. This case is a nice representation of the disease process of DVE in highly susceptible species and demonstrates the pantropic nature of DVE.
Contributing Institution:
Oklahoma State University Center for Veterinary Health Sciences
Department of Pathobiology
http://cvhs.okstate.edu/Veterinary_Pathobiology
JPC diagnosis:
1. Esophagus: Esophagitis, necrotizing, multifocal, moderate with glandular epithelial necrosis, lymphocytolysis, and occasional intranuclear and intracytoplasmic viral inclusions.
2. Liver: Hepatitis, necrotizing, multifocal, mild to moderate, with intranuclear viral inclusions.
3. Liver, hepatocytes: Vacuolar degeneration, diffuse, moderate.
4. Proventriculus: Lymphocytolysis, diffuse, mild to moderate.
JPC comment:
During case discussion, some participants noted the presence of intracytoplasmic inclusions in addition to intranuclear inclusions. As the contributor correctly identifies, this herpesvirus may result in intranuclear and intracytoplasmic viral inclusions, which have been verified as viral nucleocapsids in cytoplasmic vacuoles.3
When this disease was first described in the Netherlands in 1923, it was thought that a new and distinct virus was causing disease in ducks, and the name "duck plague" was proposed, and adopted for many years. Subsequently, the disease is now identified as Duck Viral Enteritis (DVE), to separate the disease from fowl plague.3
Of the more than 300 viral encoded miRNAs that have been catalogued, more than 95% are encoded by viruses of the herpesvirus families. A comparison between vaccine and virulent Chinese strains of duck enteritis virus show the vaccine strain had 33 encoded miRNAs, while the virulent strain encoded 39 miRNAs. The virulent strain miRNAs formed a unique regulatory pathway as compared to the vaccine strain, indicating our understanding of the true pathogenesis is incomplete.8
Other hemorrhagic and necrotic lesions in anseriforms may be caused by fowl cholera (Pasteurella multocida), Riemerella anatipestifer, duck viral hepatitis (5 different viruses), coccidiosis, Newcastle disease, avian influenza, and goose herpesvirus. Differentials for intranuclear inclusions in duck hepatocytes would also include lead toxicity (common in waterfowl), and anatid adenovirus-2.
References:
- Boulianne M. Avian Disease Manual. 7th ed. Jacksonville FL: AAAP, Inc: 2013:42-43.
- Dhama K, Kumar N, Saminathan M, et al. Duck virus enteritis (duck plague) - a comprehensive update. Veterinary Quarterly. 2017;37(1):57-80.
- Metwally SA, Cheng A. Duck Virus Enteritis (Duck Plague). In: Swayne DE ed. Diseases of Poultry, 14th Ed. Hoboken, NJ: John Wiley and Sons, Inc. 2020; 460-470.
- Miller RE, Fowler ME. Fowler's Zoo and Wild Animal Medicine. 8th ed. St. Louis, MO: Elsevier Saunders: 2015:120.
- OIE Terrestrial Manual 2012: Chapter 2.3.7 Duck Virus Enteritis. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2008/pdf/2.03.07_DVE.pdf. Retrieved 5/28/17.
- Proctor SJ. Pathogenesis of Digestive Tract Lesions in Duck Plague. Veterinary Pathology. 1975;12:349-361.
- Wobeser G. Experimental Duck Plague in Blue-Winged Teal and Canada Geese. Journal of Wildlife Diseases. 1987;23(3):368-375.
- Wu X, Jia R, Zhou J, et al. Virulent duck enteritis virus infected DEV cells generate a unique pattern of viral microRNAs and a novel set of host microRNAs. BMC Veterinary Research. 2018;14:144.