CASE III: A15-23071 (JPC 4168468)
Signalment:
Juvenile, sex unknown, discus, Symphysodon sp., fish
History:
Multiple fixed tissues were received from a discus was that was euthanized to investigate chronic low level mortalities within a group of recently acquired juvenile fish. Clinical signs within the group included poor growth and physical condition, lethargy, anorexia, and external darkening.
Gross Pathology:
No gross findings reported.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
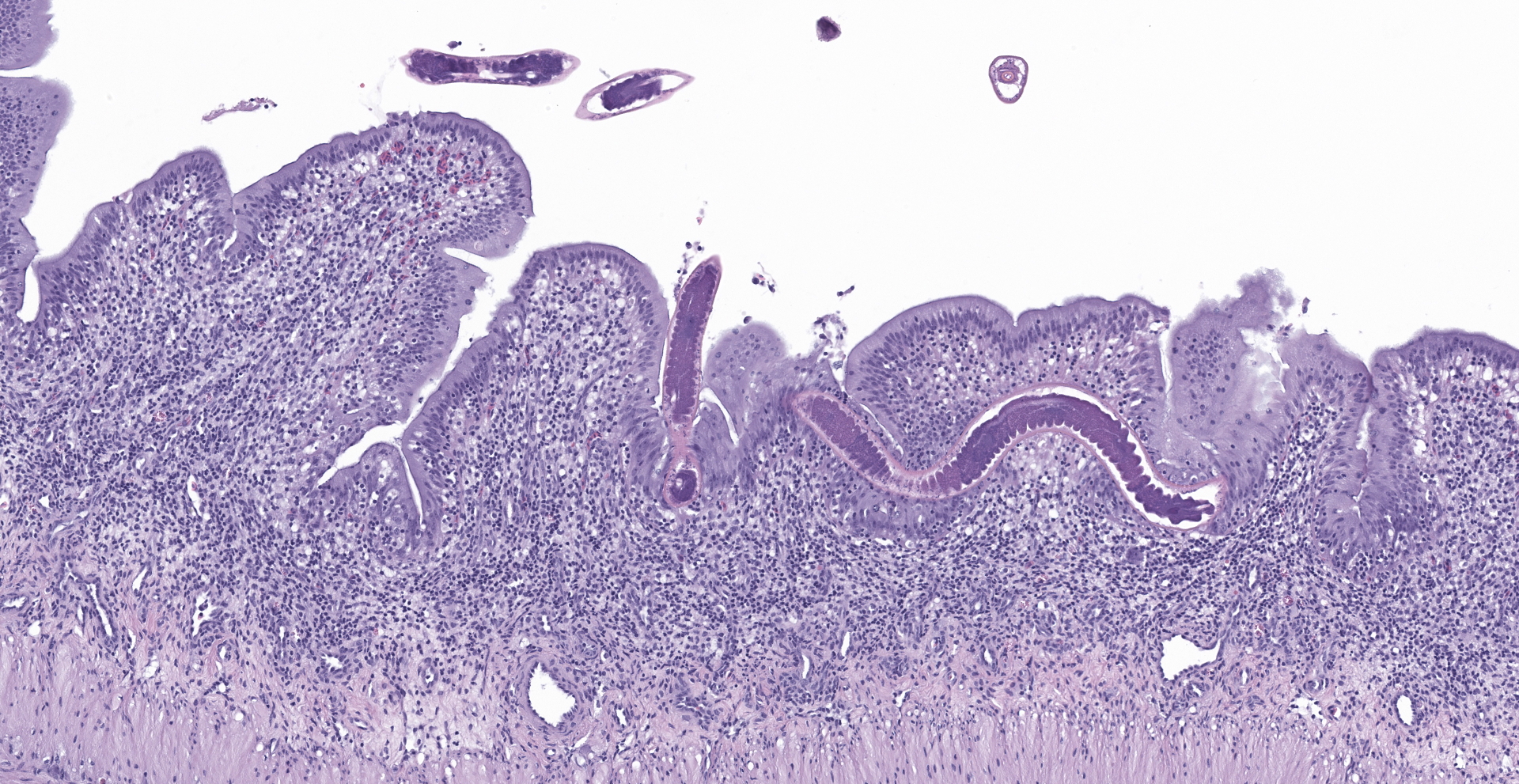
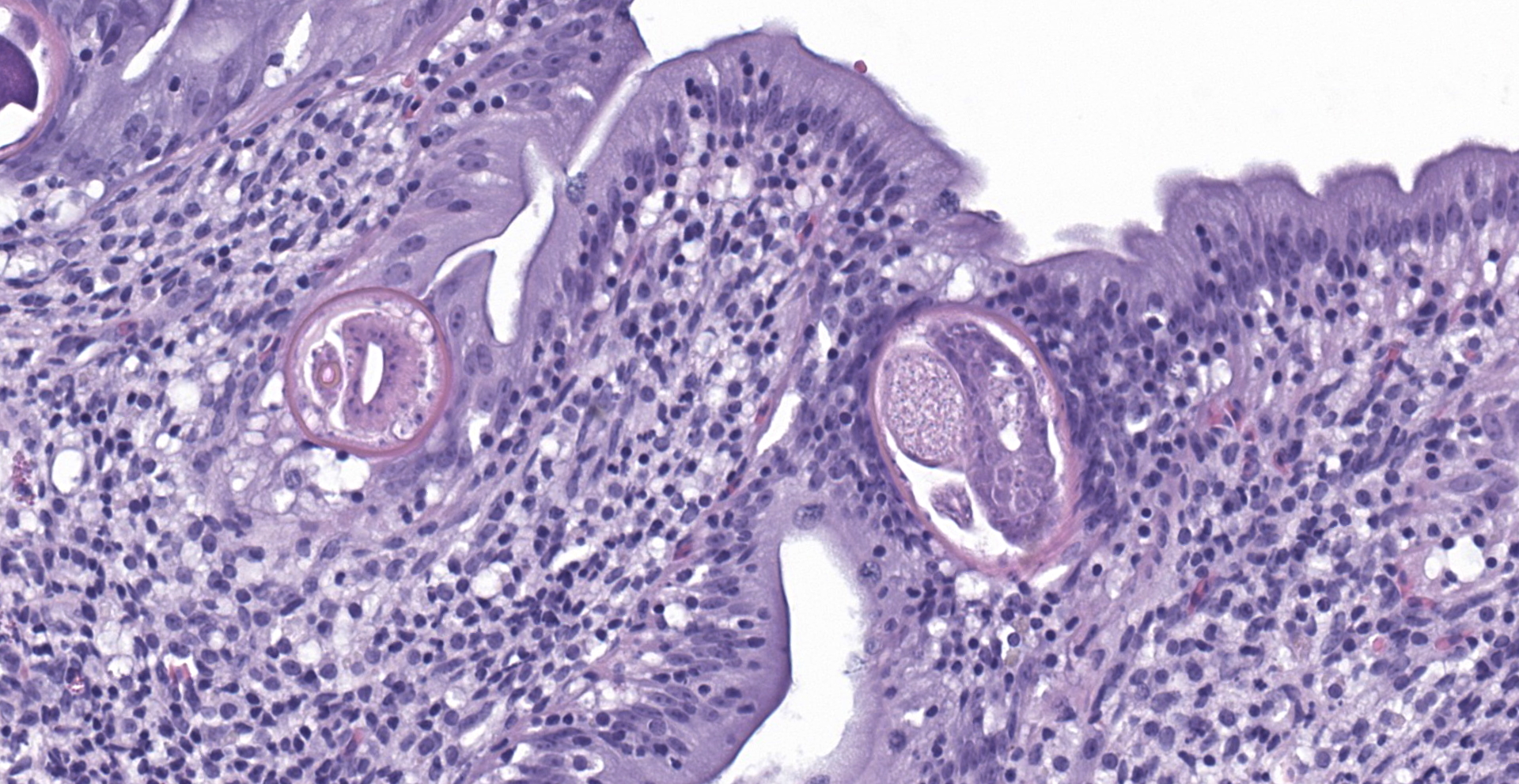
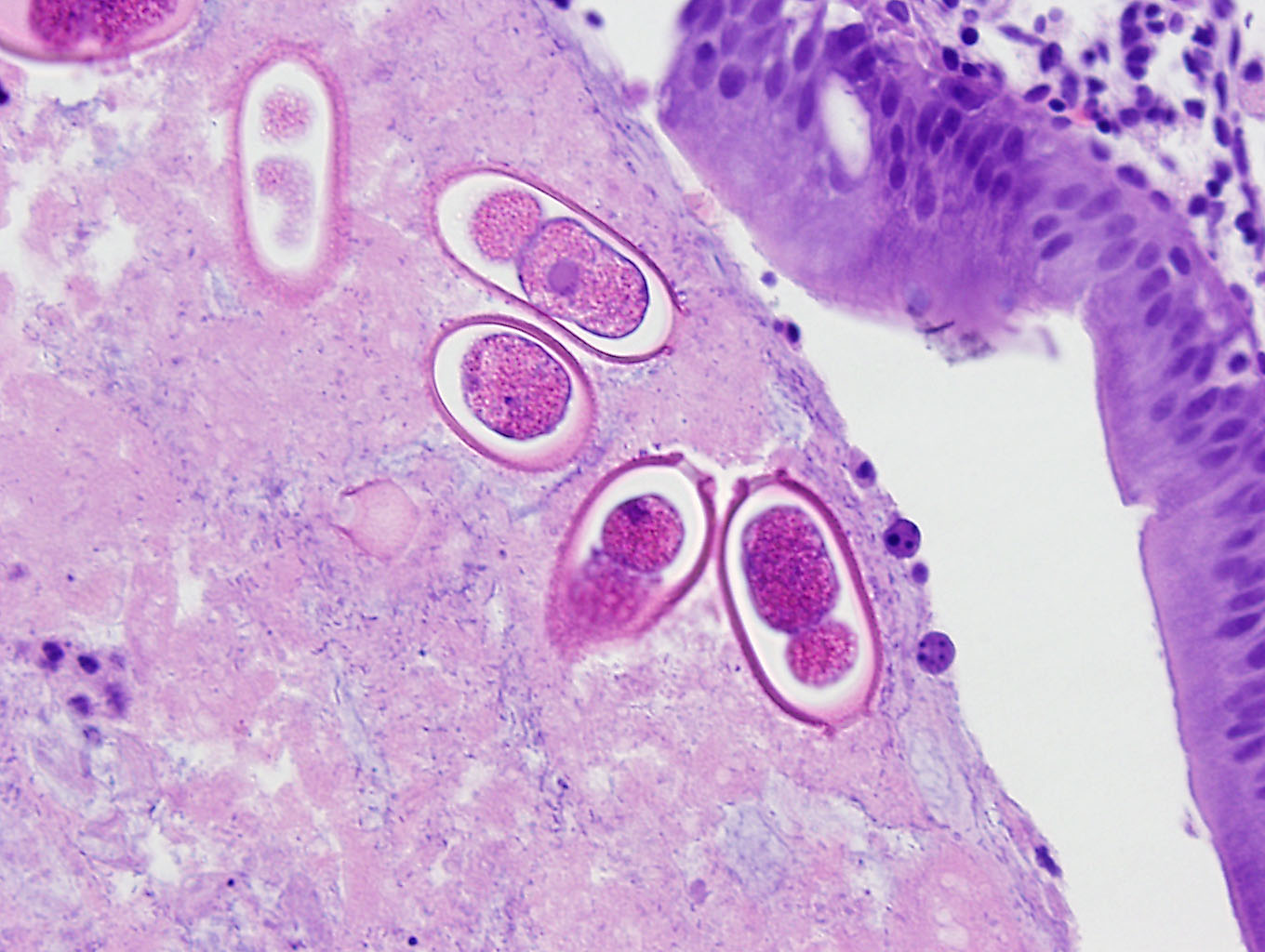
An extensive segment of intestine is characterized by widespread, variable expansion, blunting, and fusion of mucosal folds with patchy loss of the normal plicated architecture and associated epithelia. Affected mucosal surfaces are frequently disorganized with variably sized foci of epithelial attenuation, hypertrophy, and hyperplasia accompanied by scattered small foci of necrosis containing cellular debris, and widespread migration of lymphocytes across the epithelial border. The lamina propria and submucosa are markedly expanded by widespread infiltration with moderate to large numbers of lymphocytes and macrophages accompanied by scattered eosinophilic granular cells and plasma cells. Inflammatory infiltrates are accompanied by patchy edema, rare minimal foci of hemorrhage, scattered fibroblasts, and islands of entrapped epithelial cells. Vascular lumens are prominent and lined by mildly hypertrophic endothelial cells. Free within the intestinal lumen and embedded in the mucosa and submucosa are multiple longitudinal and transverse fragments of slender nematodes with a smooth cuticle, pseudocoelom, and inconspicuous coelomyarian musculature. Additional features include a prominent stichosome composed of a series of granular, amphophilic to basophilic stichocysts, and in some sections a stichosome nucleus. Multiple stages of germ cell development are evident within the same sections of ovarian and testicular tissue. Rare transverse sections of unembryonated eggs with hyaline eosinophilic shells are present in the mucosa and gut lumen. The submucosa contains a single granuloma with fragments of a degenerate worm.
Contributor's Morphologic Diagnoses:
Intestine: Enteritis, granulomatous, diffuse, chronic, severe, with intraluminal and intramural aphasmid nematodes, presumptive Capillaria pterophylli.
Contributor's Comment:
Microscopic findings in this discus fish Symphysodon spp., chronic enteritis in the presence of embedded aphasmid nematodes, are consistent with pathologic changes and morphologic features of intestinal
capillariasis, presumptively due to Capillaria pterophylli. Although often identified only to the genus level, examples of capillariasis causing losses in aquaculture operations producing ornamental fish has been reported from multiple countries and fish species. Records of C. pterophylli suggest primary hosts are South American fishes of the family Cichlidae, including angelfish Pterophyllum spp. and discus Symphysodon spp.5 However, taxonomy of the Capillaridae is disputed and some classification schemes may contain as many as 22 genera. The distinctive shape, social behavior, and array of color patterns found in discus have established them as a popular aquarium species among collectors and in competitions. Their production in several Asian countries is part of a major fish culture industry. Primary differentials for chronic ill-thrift and mortalities in discus include mycobacteriosis, spironucleosis, and cryptobiosis.
While C. pterophylli is reported to induce similar clinical signs and pathologic changes, impacts of capillariasis in small freshwater fish are best described in research colonies of zebrafish Danio rerio, where parasitism by Pseudocapillaria tomentosa is a significant cause of chronic wasting and mortality.3,5,6 Histologic changes include irregularities in the intestinal plicae, fusion of plicae, epithelial hyperplasia and dysplasia, and edema in the lamina propria. Inflammatory infiltrates composed of mixed lymphocytes, eosinophilic granular cells (mast cells), neutrophils, and fewer histiocytes further expand the lamina and submucosa.2,3,6 In zebrafish colonies, P. tomentosa, is also associated with a high incidence of intestinal carcinomas, where Mycoplasma spp. are believed to initiate tumor development that is promoted by proliferative changes induced by the nematode.2,8
The trichuroid nematodes, of which the family Capillaridae is a member, are all parasitic as adults in vertebrates. Most possess an anterior end that is narrower than the posterior end with a slender esophagus embedded in large, basophilic, glandular cells, the stichocysts, which collectively form
a stichosome. Distinct lateral chords are absent. However, hypodermal bacillary bands with associated nuclei are present but may not be visualized in all sections. Musculature is polymarian coelomyarian. In any section, ovarian and testicular tissue will contain all stages of germ cells (hologonic development). The eggs of most species have bipolar or biopercular plugs. Portions of worms are frequently found embedded within the intestinal wall.1
The life cycles of both C. pterophylli and P. tomentosa are direct by feco-oral transmission between tankmates.4,7 Movements of fish in the ornamental pet trade and the sharing of zebrafish lines, lack of quarantine, and potential for eggs to survive sanitization may contribute to spread. Due to their direct life cycles, infections cannot be contained through the elimination of intermediate hosts. However, fenbendazole, levamisole, emamectin benzoate, and ivemectin have been described as potential treatments.6 A real-time PCR assay has been developed and validated as a means of non-lethal sampling in zebrafish colonies.7
Contributing Institution:
Department of Pathology
University of Georgia
College of Veterinary Medicine
501 DW Brooks Drive
Athens, GA 30602
https://vet.uga.edu/education/academic-departments/pathology/
JPC
Diagnosis:
1. Intestine: Enteritis, lymphohistiocytic, diffuse, moderate, with intramucosal aphasmid adults and luminal eggs.
2. Stomach, submucosa: Granulomas, few, with edema.
JPC Comment:
Capillaria pterophylli was first described by Heinze (1933) in two species of angelfish (Pterophyllum scalare and P. eimekei) native to South America, which are commonly found in aquariums. Fish of the family Cichlidae (Pterophyllum and Symphysodon) originating from South America are thought to be the primary hosts although other species of aquarium fish from different families and geographic regions have also been infected, including Puntius tetrazona (Cyprinidae) and Colisa lalia (Belontiidae). Notably, the majority of reported infected fish are not directly imported, suggesting the life-cycle can be completed in aquarium conditions.4
As noted by the contributor, Pseduocapillaria tomentosa is a common cause of capillariasis in zebrafish (Danio rerio). This species serves as an important animal model used in various research studies including genetics, development, infectious disease, and toxicology. Compared to other laboratory animals, such as murine models, utilization of zebrafish in research is a relatively recent endeavor. Consequentially, infectious diseases are a commonly faced issue in the research setting with an impact on not only population management but the value of research involving their use can also be diminished as a result. Modern research facilities frequently utilize multiple biosecurity measures such as quarantine rooms, routine visual assessment for diseased fish, and screening sentinels and culled older fish for pathogens using histology and PCR/qPCR.7
Development of non-lethal screening assays that assess tank water for pathogens have generated considerable interest concerning the infection status of individual tanks. The detection of primary pathogens in tank water such as Pseudoloma neurophilia, Mycobacterium marinum, M. chelonae and M. haemophilium is a direct indication of infected (although often asymptomatic) fish given these pathogens are not found in the environment unless the fish are infected.7
Development of a qPCR assay capable of detecting Pseduocapillaria tomentosa using tank water as well as pooled fish samples was recently described with a limit of detection less than one individual nematode (0.01 nematode) in the pooled samples.7 However, sensitivity was degraded in water samples due to contaminants such as fish DNA, algae, food residue, fish waste, other free-living organisms, and other organic and inorganic compounds such as humic acid. Given infection with this nematode is associated with chronic inflammation, emaciation, and predisposition to intestinal neoplasia, use of this assay may be an effective tool toward reducing confounding lesions and development of pathogen free piscine models in research over the long-term.7
References:
1. Gardiner CH, Poyton SL. An Atlas of Metazoan Parasites in Animal Tissues. Armed Forces Institute of Pathology; 2006.
2. Gaulke CA, Martins ML, Watral VG, et al. A longitudinal assessment of host-microbe-parasite interactions resolves the zebrafish gut microbiome's link to Pseudocapillaria tomentosa infection and pathology. Microbiome. 2019:7:10 doi.org/10.1186/s40168-019-0622-9.
3. Kent ML, Sanders JL, Spagnoli S, Al-Samarroe CE, Murray KN. Review of diseases and health management in zebrafish Danio rerio (Hamilton 1822) in research facilities. J Fish Dis. 2020;43:637-650.
4. Moravec F, Gut J. Morphology of the nematode Capillaria pterophylli Heinze, 1933, a pathogenic parasite of some aquarium fishes. Folia Parasitol (Praha). 1982;29:227-231.
5. Moravec F, ProkopičJ, Shlikas AV. The biology of the family Capillariidae Neveu-Lemaire, 1936. Folia Parasitol (Praha). 1987; 34:39-56.
6. Murray KN, Peterson TS. Pathology in practice. JAVMA. 2015;246:201-203.
7. Ahmad F, Fazili AF, Sofi OM, Sheikh BA, Sofi TA. Distribution and pathology caused by Bothriocephalus acheilognathi Yamaguti, 1834 (Cestoda: Bothriocephalidae): a review. Rev Vet. 2018;29(2):142-149.
8. Norris L, Lawler N, Hunkapiller A, et al. Detection of the parasitic nematode, Pseudocapillaria tomentosa, in zebrafish tissues and environmental DNA in research aquaria. J Fish Dis. 2020;43:1081-1095.
9. Schaaf RM, Sharpton TJ, Murray KN, Kent AD, Kent ML. Retrospective analysis of the Zebrafish International Resource Center diagnostic data links Pseudocapillaria tomentosa to intestinal neoplasms in zebrafish Danio rerio (Hamilton 1822). J Fish Dis. 2020;43:1459-1462.