Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 8
October 24, 2018
CASE II: D15-060481 (JPC 4082891-00).
Signalment: Hatch-year female bald eagle (Haliaeetus leukocephalus)
History: This animal was admitted to The Raptor Center (TRC) of the University of Minnesota on December 13, 2015. It was recumbent and had head tremors. The animal was severely dyspneic. Small radio-dense fragments were detected in the ventriculus by x-ray. The animal had a PCV of 27% (considered to be anemic). It was euthanatized the same day after blood analysis revealed a markedly elevated lead level (3.3ppm).
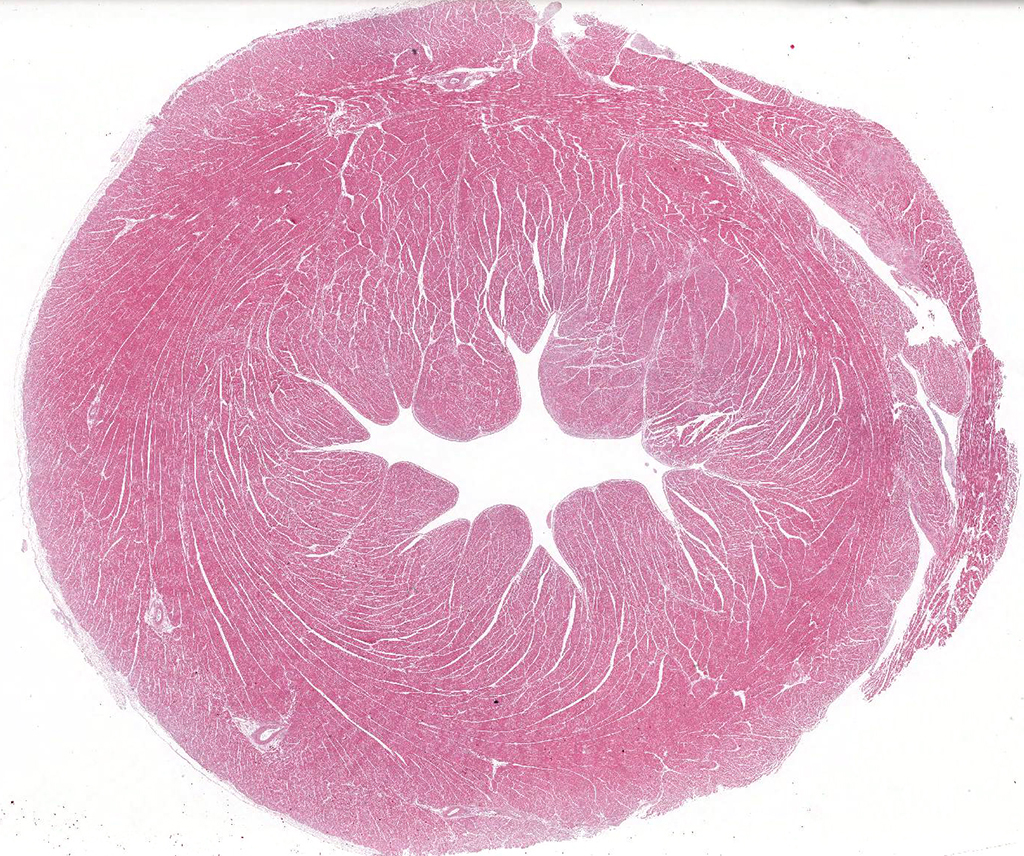
Gross Pathology: The bird was in a good nutritional state. It was anemic. The pericardial sac contained 45ml of clear yellowish watery fluid with a small amount of flocculent material (heart weight: 53g). The myocardium of the left and right ventricle was multifocally beige discolored. Approximately 30% of the myocardium, particularly subjacent to the endocardium of the left ventricle, were affected. The proventriculus and ventriculus contained multiple small metallic fragments.
Laboratory results: None performed.
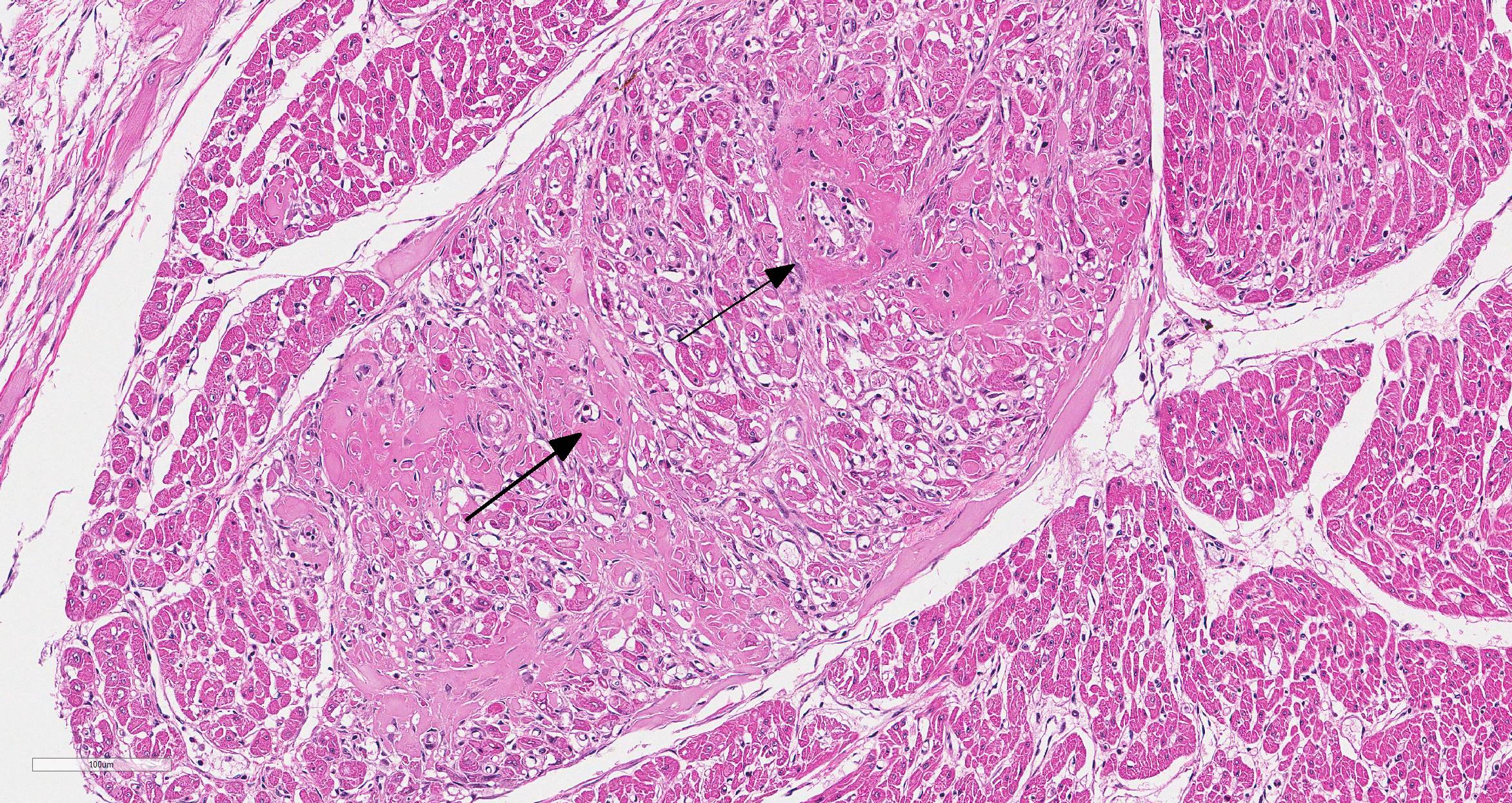
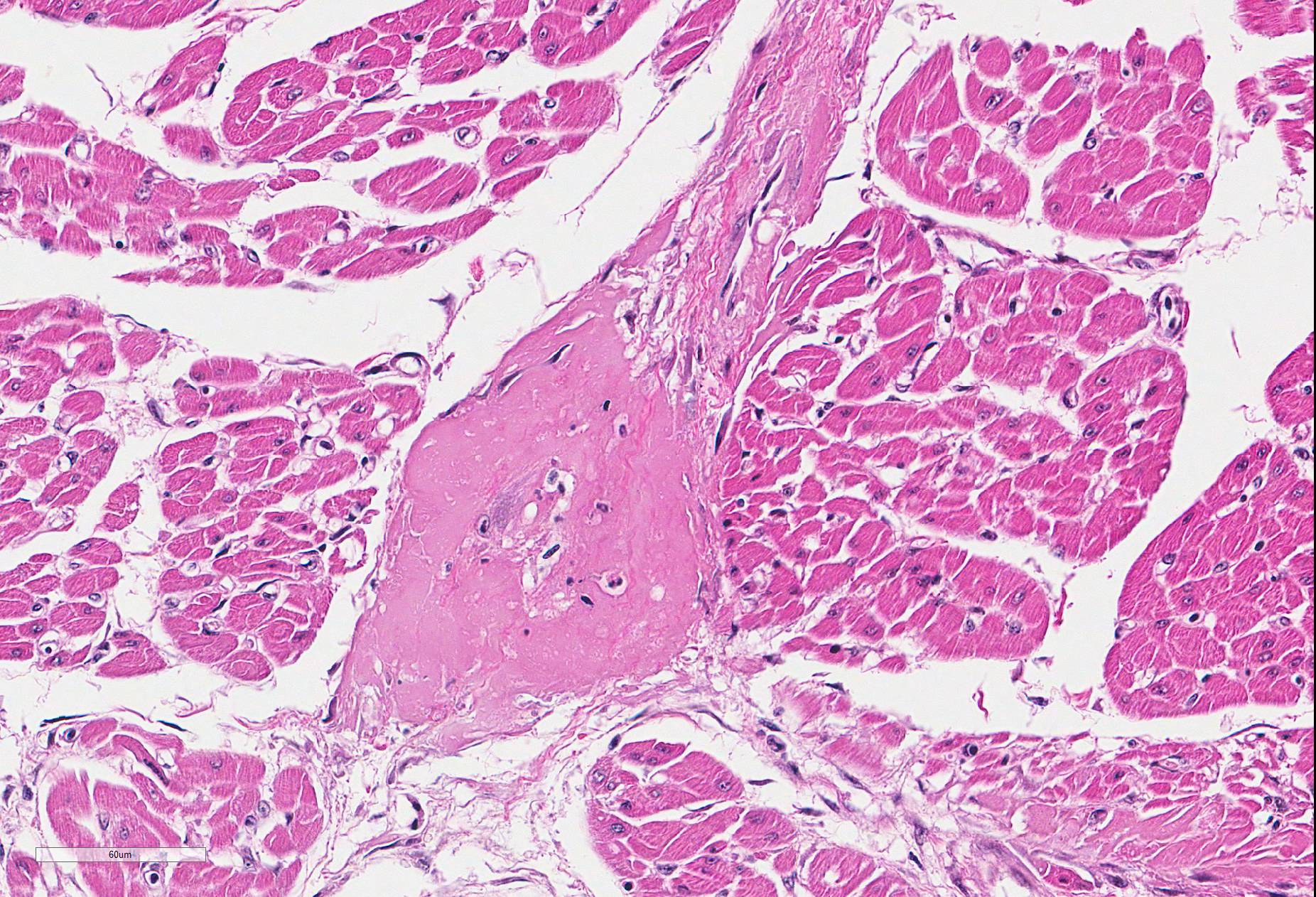
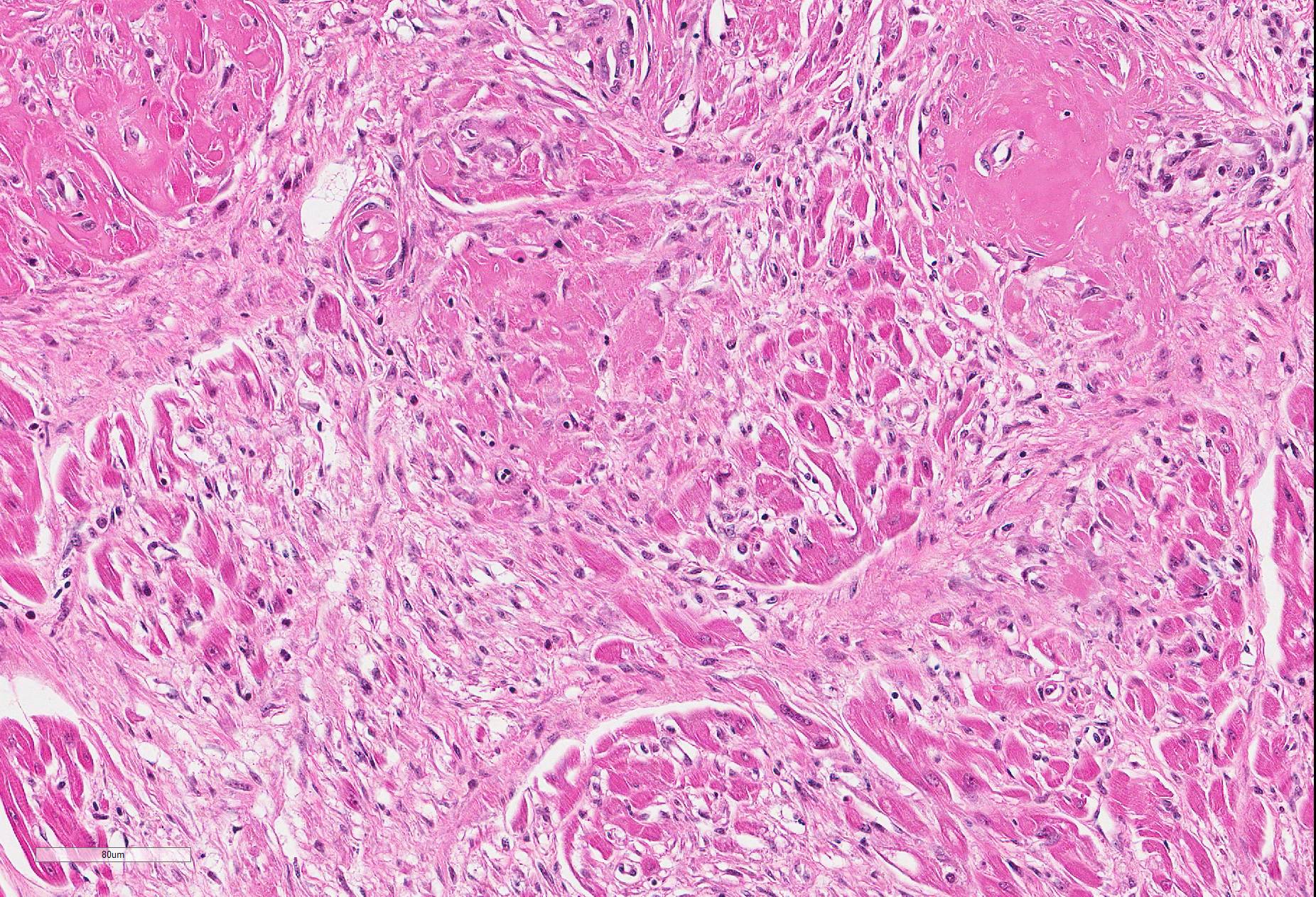
Microscopic Description: Heart (left ventricle) – The lesions are most pronounced in one papillary muscle of the left ventriculus but to a lesser degree are present at other sites of the myocardium (eg subepicardial myocardium). Cardiomyocytes in numerous fascicles of the aforementioned papillary muscle are shrunken and slightly hypereosinophilic containing homogenous (“hyalinized”) material. Occasional pyknotic nuclei of cardiomyocytes are present. The number of cardiomyocytes in some fascicles appears to be reduced and the endomysium appears more prominent and slightly vacuolated. The number of fibrocytes in the endomysium is slightly increased in few locations with subtle deposition of collagenous matrix. In more affected fascicles, groups of cardiomyocytes are replaced by pools of homogenous eosinophilic acellular (“hyalinized”) material. The smooth muscle cells in the media of occasional small to medium caliber myocardial arteries are mildly vacuolated. The endothelial cells of these arteries are slightly hypertrophied. In few arteries, homogenous eosinophilic (“hyalinized”) material replaced the media or portions of the media and adventitia.
Contributor’s Morphologic Diagnoses:
Heart:
a.fibrinoid necrosis of small and medium caliber myocardial arteries, multifocal, acute
b. myocardial necrosis, multifocal, moderate, acute.
c. myocardial fibrosis, multifocal, mild.
Contributor’s Comment: The cardiac lesions in this case are most consistent with a degenerative or toxic cardiomyopathy. The result of the blood lead analysis is diagnostic of lead poisoning. The blood lead level of 3.3ppm is high considering that bald eagles with levels above 1ppm have a poor prognosis for survival even when treated by chelation and are frequently euthanized at admission.12 The good nutritional state of the bird at the time of death suggests a fairly acute course of the disease. Accordingly, the myocardial lesions were dominated by rather acute changes with only subtle early myocardial fibrosis.
Lead toxicity is a common cause of death in scavenging raptors such as eagles and vultures as well as waterfowl worldwide. Cardiac lesions including hydropericardium, myocardial necrosis, fibrinoid necrosis of myocardial vessels and myocardial fibrosis have been described in bald eagles.2,6,7,10 Approximately 36% of lead intoxicated eagles submitted to the National Wildlife Health Center (Madison, Wisconsin, USA) had microscopic cardiac lesions. Myocardial infarction with angiopathy is also common in waterfowl with fatal lead intoxication.4. Besides the cardiac lesions, tubulonephrosis and brain lesions, including hemorrhages and parenchymal necrosis, have been reported in eagles with plumbism.8,10 Anemia, bile stasis and bile staining of gastrointestinal mucosa are considered to be common gross findings in lead intoxicated eagles but are unspecific as to the cause and highly subjective findings.7 Lead is known to have a wide range of pathophysiologic effects. Lead interferes in the avian host with sulfhydryl-dependent enzyme function (eg, delta aminolevulinic acid dehydrase); mimics calcium hereby interfering with neurologic function and mitochondrial respiration; and adversely affects DNA and RNA synthesis.3 However, the exact pathogenesis of the lead-associated fibrinoid vascular necrosis, cardiomyocyte degeneration, and myocardial fibrosis is uncertain.
While waterfowl pick up lead pellets (accumulated in lakes after hundreds of years of hunting with lead-based ammunition and ongoing illegal use of lead-based ammunition), lead sinkers, etc. while dabbling, lead exposure in eagles and scavenging birds occurs almost exclusively by ingestion of carcasses and offal of animals (upland birds and ungulates) killed with lead-based ammunition.1 Despite the ban of lead-based ammunition for waterfowl hunting in the USA and Canada in the seventies, the flow of cases of lead-intoxicated scavenging birds to wildlife rehabilitation centers and diagnostic labs has not slowed.5 Extending the ban of lead-based ammunition to hunting of upland birds, wild turkey and ungulates would prevent lead poisoning in scavenging birds.1
Contributing Institution:
University of Minnesota Veterinary Diagnostic Laboratory
http://www.vdl.umn.edu
JPC Diagnosis: Heart, small- and medium-sized arteries: Fibrinoid necrosis, multifocal, severe, with myofiber degeneration, necrosis and atrophy and marked myocardial fibrosis.
JPC Comment: Lead is a well-known and potent environmental and industrial contaminant. It is widely distributed in the body and stored in the kidney, liver, and bone (where it may reside for up to 35 years!). Its effects in the hematopoietic, urinary, musculoskeletal, and nervous systems are well known, even if many gaps yet persist in our knowledge of its pathophysiology in these systems.
One of the more recent areas of investigation in lead toxicosis are its effects upon the cardiovascular system. Damage to the vascular system as a result of lead intoxication is likely multifactorial, and lead exposure in humans has been identified in an increased incidence of cardiovascular disorders such as hypertension, organic heart disease, and peripheral arterial diseases, including atherosclerosis.9 Research in animal models and human populations has also shown a direct and causal relationship between low-level lead exposure and hypertension. 11
At the cellular level, lead is a major driver in free radical damage affecting both endothelial cells and smooth muscle cells. Entering the cells through normal calcium channels, lead inhibits endoplasmic reticulum (ER) Ca2+-ATPase, resulting a release of calcium into the cytoplasm, and triggering ER stress as a result of release of calcium-dependent signaling and chaperone proteins contained within the ER.11 In addition, it can bind directly to the calcium-binding protein, glucose-regulated protein 78 (GRP78), another ER-based chaperone protein involved in ER stress. 11 Activation of these proteins results in elevated levels of reactive nitrogen species in damaged cells and measurable increases in the cytotoxic effects of lead. In vitro studies of cardiofibroblasts has also shown that administration of lead induces autophagy (a protective response) through inhibiting the mammalian target of rapamycin complex 1 (mTORC1) pathway.13While speculative, the vascular changes seen in this case suggest a correlation between hypertension, endothelial damage and the toxic principles of lead at the cellular level. The arterial lesions seen in this case are similar to those of polyarteritis nodosa (PN), a change often seen in hypertensive rat models. A vicious cycle of endothelial damage and hypertension may result in similar lesions. (One difference between this lesion and PN, however, is the lack of cellular proliferation in the adventitia associated with many cases of PN).
In this section, myocardial damage appears most severe in the areas of vascular necrosis, suggesting a cause-and-effect scenario, bolstered by the concurrent presence of myofiber degeneration, necrosis, and fibrosis, denoting a polyphasic timeline consistent with a toxic vascular injury. It is certainly possible that a similar direct effect of lead on cardiocytes, in addition to that seen in the vessels may have contributed to the widespread myocardial damage in this section.
The most diagnostic tissue to submit for lead toxicosis post mortem is the liver. Although lead accumulates in bone, bone levels of lead represent a lifetime accumulation of this metal and therefore are difficult to correlate to point in time toxicosis if acute toxicity is suspected. As stated by the contributor, exposure to lead in these animals is via ingestion. Although embedded lead shot is common in wildlife, intramuscular lead does not readily dissolve, and poses little risk for toxicosis. It is unusual that presumptive lead fragments were identified in the proventriculus and ventriculus in this case as birds of prey with plumbism frequently cast out metallic fragments prior to death. An additional histologic finding of lead toxicosis, though rare, is intranuclear, acid-fast inclusions in renal tubular epithelial cells. These lead inclusions have not been reported in eagles or California condors, but have been seen in turkey vultures and Andean condors.
Attendees discussed a number of differential diagnoses for this case including West Nile virus, capture myopathy, nutritional imbalance of Vitamin E and selenium, and even electrocution (which may result in hemopericardium at necropsy). The absence of significant inflammation argues against West Nile Virus infection (although interestingly, the heart, brain, and eye are preferentially affected - the same triad of organs that exhibit vasculitis in lead–intoxicated birds.) The polyphasic nature of the lesion and concomitant vasculitis argues against the possibility of capture myopathy, and vasculitis is uncommon in most species with Vitamin E/Se imbalance (with the possible exception of swine).
It is not uncommon for particulate matter containing lead to be absent from poisoned birds at autopsy. When submitting avian tissues for lead measurement, most reference ranges are based on liver accumulation, which would best represent acute lead storage (as opposed to bone levels, which may reflect a lifetime accumulation of lead). When searching histologically for evidence of lead toxicosis, kidneys are a good choice for the identification of intranuclear inclusions.
References:
- Clark AJ, Scheuhammer AM. Lead poisoning in upland-foraging birds of prey in Canada. Ecotoxicol. 2003;12: 23-30.
- Franson RE, Russell JC. Causes of mortality in eagles submitted to the National Wildlife Health Center 1975-2013. Soc. Bull. 2014;38: 697-704.
- Haig SM, D’Elia J, Eagles-Smith C, Fair JM, Gervais J, Herring G, Rivers JW, Schulz JH. The persistent problem of lead poisoning from ammunition and fishing tackle. The Condor 2014;116: 408-428.
- Karstad L. Angiopathy and cardiomyopathy in wild waterfowl from ingestion of lead shot. Med. 1971;35: 355-360.
- Kramer JL, Redig PT. Sixteen years of lead poisoning in eagles: 1980-95: an epizootiologic view. Raptor Res. 1997;31: 327-332.
- Langelier KM, Andress CE, Grey TK, Wooldridge C, Lewis RJ, Marschetti R. Lead poisoning in bald eagles in British Columbia. Vet. J. 1991;32:108-109.
- LaDouceur EE, Kagan R, Scanlan M, Viner T. . Chronically embedded lead projectiles in wildlife: a case series investigating the potential for lead toxicosis. J Zoo Wild Med 2015; 46(2):438-442.
- Locke LN, Thomas NJ. Lead poisoning in waterfowl and raptors. In: Fairbrother N, Locke LN, Hoff GL, eds. Non-Infectious Diseases of Wildlife. 2nd Iowa State University Press. Ames, Iowa, USA; 1996: 108-117.
- Nicolas de Francisco O, Feeney D. Armien A, Wunschmann A, Redig PT. Correlation of brain magnetic resonance imaging of spontaneously lead poisoned bald eagles (Haliaeetus leucocephalus) with histologic lesions: a pilot study. Vet. Sci. 2016;105: 236-242.
- Patrick, N. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Alt Med Rev 2006; 11(2):114-126.
- Pattee OH, Wiemeyer SN, Mulhern BN, Sileo L, Carpenter JW. Experimental lead shot poisoning in bald eagles. Wildl. Manage. 1981;45: 806-810.
- Shinkae Y, Kaji T. Cellular defense mechanisms against lead toxicity in the vascular system. Biol Pharm Bull 2012; 35(11): 1885-1891.
- Stauber E, Finch N, Talcott PA, Gay JM. Lead poisoning of bald (Haliaeetus leucocpehalus) and golden (Aquila chrysaetos) eagles in the the US inland pacific northwest region – an 18–year retrospective study: 1991-2008. Avian Med. Surg. 2010;24: 279-287.
- Sui L, Zhang RH, Zhang P, Yunj, KL, Zhang HC, Liu L, Hu MX. Lead toxicity induces sutophagy to protect against cell death through the mTORC1 pathway in cardiofibroblasts. Biosci Rep 2015; 31;35(2). pii: e00186
- Wunschmann A. Birds of Prey. In: Pathology of Wildlife and Zoo Animals. Terio K, McAlooseD , St. Leger J, eds. London, Academic Press, pp. 717-740