Signalment:
Research and Diagnostic Laboratory at South Dakota State University by the South Dakota Department of Game Fish and Parks for diagnostic workup due to concern with possible viruses that attack species such as koi, carp, and goldfish.
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Etiology: Cyprinid Herpesvirus 2, confirmed by PCR.
Lab Results:
Condition:
Contributor Comment:
JPC Diagnosis:
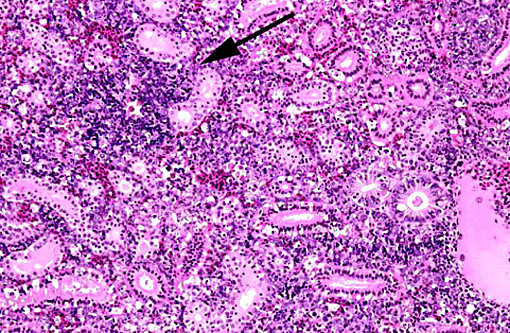
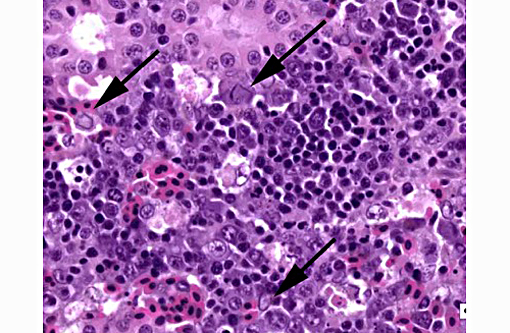
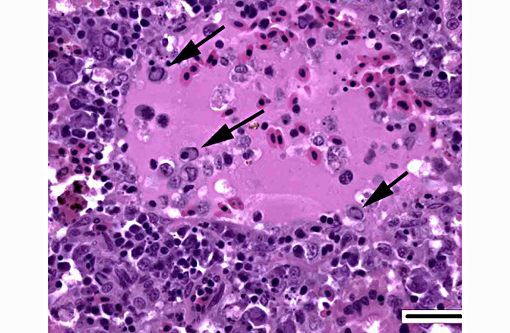
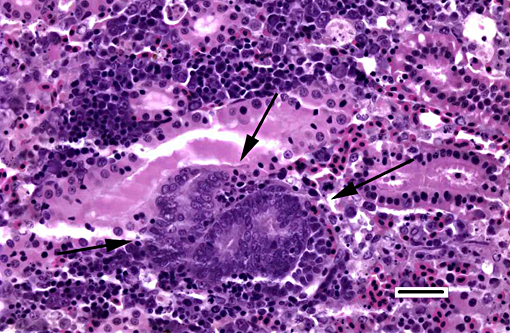
1. Kidney, hematopoietic tissue: Necrosis, diffuse, with moderate hyperplasia and numerous intranuclear inclusion bodies.
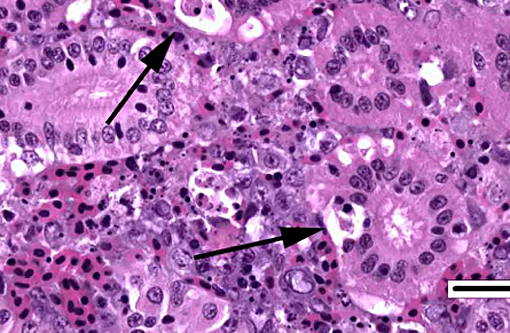
2. Kidney, tubules: Degeneration, necrosis and loss, diffuse, with rare intranuclear inclusion bodies.
Conference Comment:
The three cyprinid herpesviruses are closely related to anguillid herpesvirus 1; all 4 viruses are grouped within the genus Cyprinivirus. Cyprinid herpesvirus-3 (CyHV-3) is highly contagious and is also termed koi herpesvirus disease. It is an OIE reportable disease and is also present throughout the world due to global fish trade; it can be devastating to the production of koi and common carp. Characteristic signs of disease include erratic swimming, gasping for air, poor appetite, discoloration and fin erosions. Histologic lesions include gill, liver and renal necrosis with intranuclear inclusion bodies.
Hyperplasia of gastric gland epithelium, intestinal villi, and respiratory cells may also be seen, resulting in lamellar fusion. Survivors of CyHV-3 outbreaks and other unaffected fish species can act as carriers. The virus is transmitted horizontally and fish density may play a role in severity and spread of infection in an outbreak. Cyprinid herpesvirus-1 causes papillomatous skin lesions in infected koi; the virus is lethal in young fish, but is generally not lethal in adult koi.(4)
Consistent histopathologic findings in CyHV-2 infection include necrosis of hematopoietic tissue in the spleen and/or kidney (necrosis may or may not be present in both locations), with or without the presence of characteristic herpesviral inclusion bodies. Other reported microscopic findings include: branchial epithelial hyperplasia, necrosis(1,2) and hypertrophy; necrosis and inflammation in the intestine; and lesions in the heart.(1) Viral DNA has been interstitium with eosinophilic cellular and demonstrated in subclinically affected animals with the presence of single cell necrosis in hematopoietic tissue, suggesting a possible role of latent infection in the pathogenesis of this disease.(2)
The most prominent lesion in these sections is the diffuse necrosis within the expanded renal hematopoietic tissue, and the presence of numerous prominent intranuclear inclusions within hematopoietic precursor cells and occasionally within renal tubule epithelium. The tunica media of few vessels is discontinuous and infiltrated by low numbers of inflammatory cells, however, the moderator pointed out that this is likely an innocent bystander lesion in areas of necrosis and not a true vasculitis. There is multifocal renal tubule epithelial degeneration, necrosis and rare regeneration. Herpesviral intranuclear inclusion bodies are also present within circulating leukocytes, which can be seen in the lumen of several small vessels. The moderator also discussed the presence of mild compensatory hematopoietic tissue hyperplasia and erythrophagocytosis.
References:
1. Boitard PM, Baud M, Labrut S, Boisseson C de, et al. First detection of Cyprinid Herpesvirus 2 (CyHV-2) in goldfish (Carassius auratus) in France. J Fish Dis. 2015; July, 14; E pub ahead of print. DOI 10.1111; jfd.12400.
2. Giovannini S, Vergman SM, Keeling C, Lany C, et al. Herpesviral Hematopoietic Necrosis in Goldfish in Switzerland: Early Lesions in Clinically Normal Goldfish (Carassius auratus). Vet Pathol. 2015; Nov 9; Online first. DOI: 10.1177/0300985815614974.
3. Goodwin AE, Khoo L, LaPatra SE, Bonar C, et al. Goldfish hematopoietic necrosis Herpesvirus (Cyprinid herpesvirus 2) in the USA: molecular confirmation of isolates from diseased fish. J Aquat Anim Health. 2006;18:1118.