Signalment:
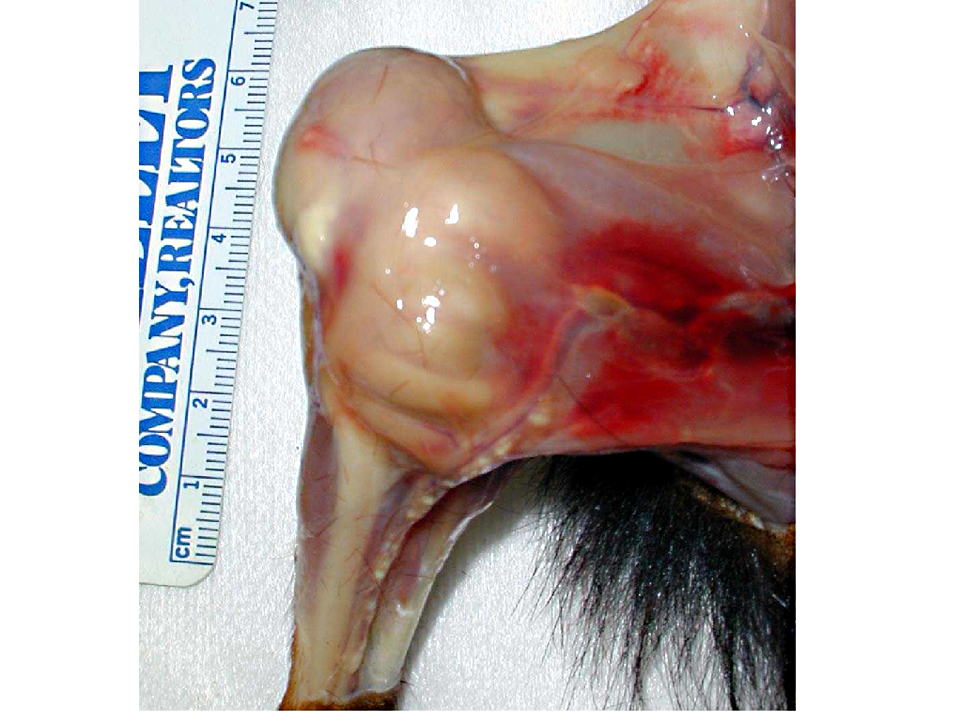
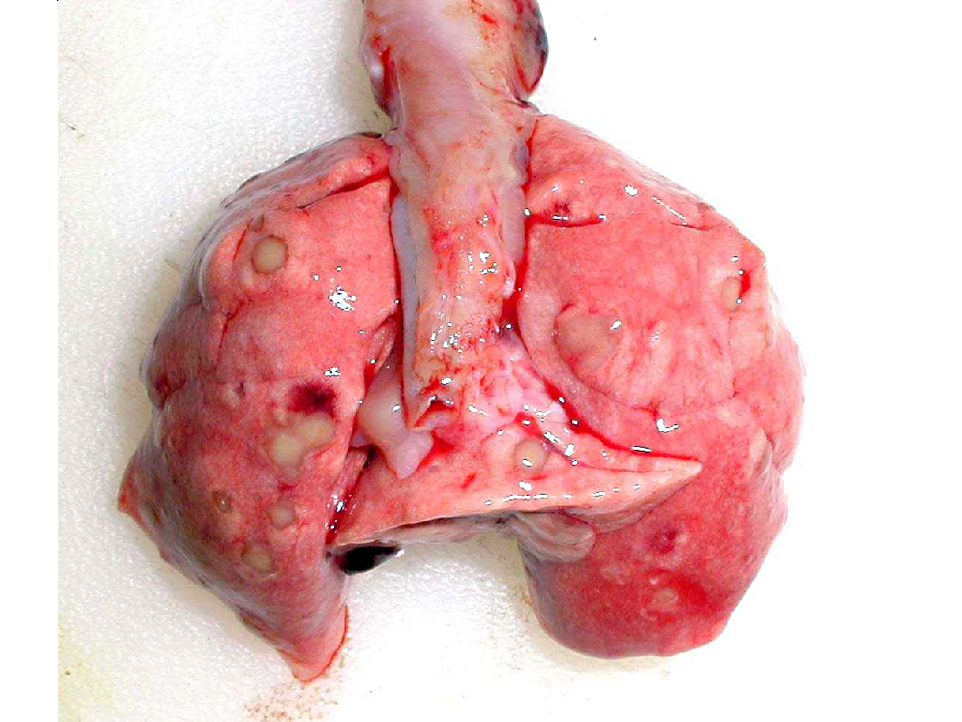
Gross Description:
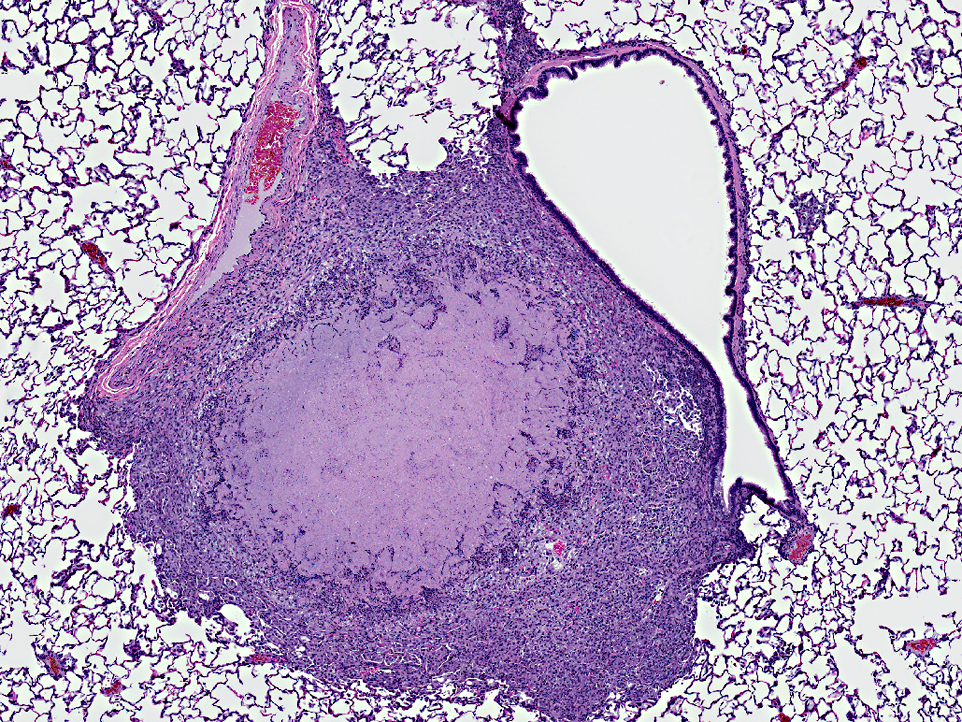
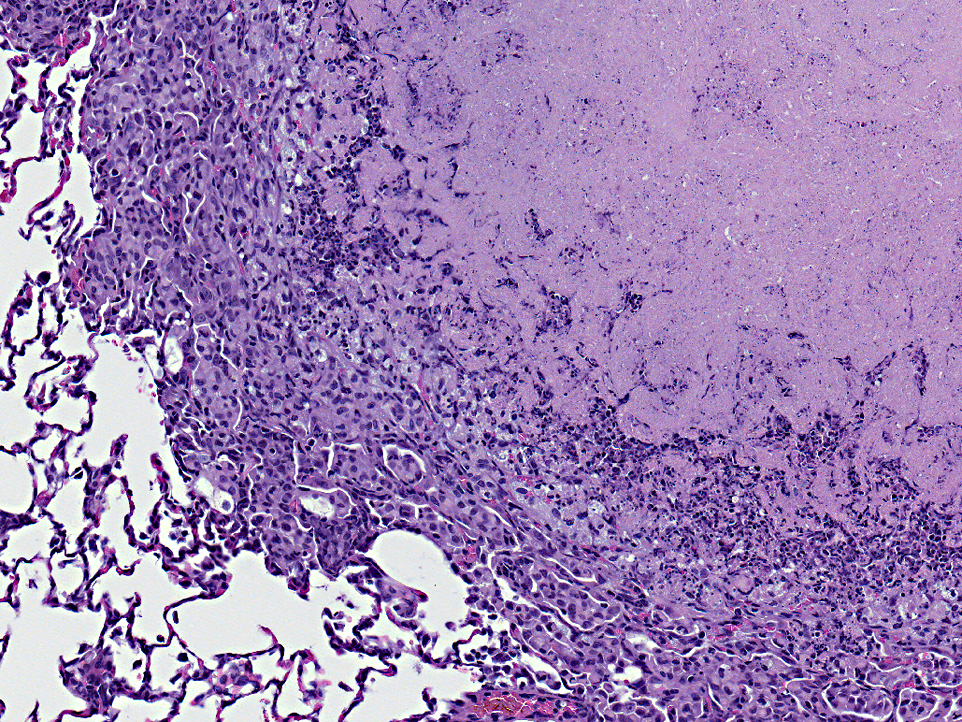
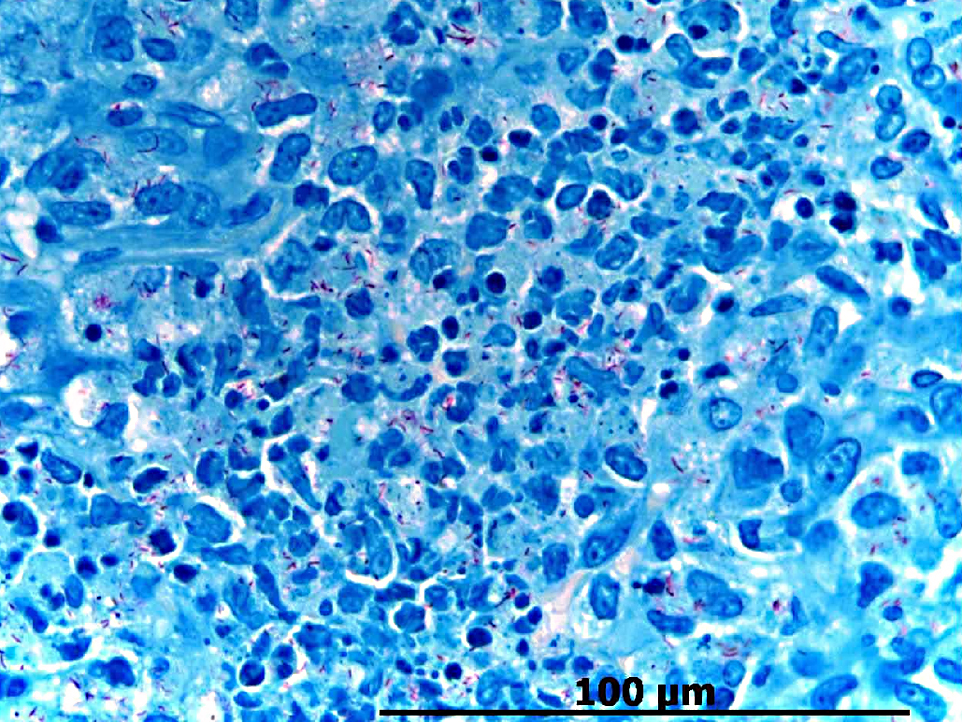
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Microbiology Culture:
Right stifle joint and lung: Mycobacterium spp.
PCR:
A 100% identity for Mycobacterium intracellular
Condition:
Contributor Comment:
Mycobacterium avium complexes (MAC) are a group of nontuberculous mycobacteria characterized as gram-positive, aerobic, non-motile, acid-fast rods. M. avium and M. intracellulare are the most notable species within the group.(30) Because M. avium ssp. avium and M. intracellulare are difficult to distinguish on the basis of phenotypic characteristics, the term M. avium complex is used when referred to these organisms. The mycobacterium now designated as M. intracellulare was first cultured in 1969 from the sputum of tuberculosis patients in the Battey State Hospital of Rome, Georgia and was originally referred to as Battey bacillus.(25) In 1971, the nontuberculosis mycobacteria were divided into three groups; based on their association with human disease, production of yellow or orange pigment, and their rate of growth. The photochromogens contain 3 species that develop yellow or orange pigment when exposed to light. Examples include M. kansasii, M. marinu, and M. simiae. The scotochromogens, including M. gordonae, M. szulgai and M. scrofulaceum, form orange-yellow pigment in the dark. The unpigmented M. avium complex (MAC), M. xenopi, and M. malmoense have been classified as nonphotochromogens.(25) Based on glycolipid typing MAC has been further subdivided into 28 serotypes.(28) Serotypes 1 to 6 and 8 belong to M. avium and serotypes 7, 12, 14, 16, and 18 to M. intracellulare. Rodents, insectivores, domestic animals and humans can be a reservoir of mycobacteria. The occurrence of mycobacteria in rodents was first reported by Wells and Oxon5, with a prevalence of 9 to 31% of Mycobacterium microti in rodents. Mycobacteriosis in shrews was also demonstrated by the isolation of the organism from tuberculous pathomorphological changes in the lungs, spleen, liver, kidneys and lymph nodes in 8 of 500 common shrews (Sorex araneus) caught in 1946 in Great Britain.(5) Mycobacteria are found in the feed and bedding, as well as the droppings of livestock.(9). Insectivores and small rodents commonly come into contact with mycobacteria via plants, animal foods, watery environments or contact with birds or humans infected with MAC. At the same time, shrews can easily get inside human or livestock housing and contaminate the environment with their droppings. Mycobacterium avium-intracellulare is a common cause of disseminated disease among patients with human immunodeficiency virus (HIV) infection.(3) In a recent study, Voles (Microtus pennsylvanicus) infected with M. bovis were capable of disseminating the organism via their feces. Both M. intracellulare and M. avium are capable of gastric transit in animals without destruction due to their resistance to acid. In this way they can be spread over long distances with the migration of the animal.(6) Also, there are several other possible routes of mycobacterial transmission: a) through direct contact with rodent excreta, b) ingestion of food or water contaminated with rodent excreta, c) ingestion of the animal itself, d) inhaling aerosolized rodent excreta, or e) rodent bites, or through ectoparasites.(9)
Depending on the route of infection, affected mammals can present with local cutaneous disease or systemic signs related to the alimentary, and/or respiratory tracts. Typically, disseminated MAC disease begins as a localized process that progress rapidly to include numerous organ systems (i.e. pulmonary, central nervous system, lymphatic, gastrointestinal, and musculoskeletal system).(2-4,7,9-12,14,16-19,21-22,24) The common clinical signs of a MAC infection in immune compromised humans are respiratory distress(19,26), anemia, chronic diarrhea, weight-loss(14), lymphadenitis(19,23), lethargy, hepatosplenomegaly, and arthritis.(29,31) MAC infections must be differentiated from M. tuberculosis or M. bovis, and other nontuberculous mycobacterial infections, as well as histoplasmosis, cryptococcosis, coccidioidomycosis, and blastomycosis. The rapid speciation and prevalence estimation of these infecting organisms is desirable because of the public health concerns associated with M. avium intracellulare, M. bovis, and M. tuberculosis infections.(27) Thus, laboratory diagnoses by culture, PCR, and /or histopathology are the traditional methods of testing. A positive culture of MAC from normally sterile sites (blood, bone marrow, lymph node, or liver biopsies), should be considered diagnostic of disseminated disease.(21)
In this case, one of three possible mechanisms probably explains the occurrence of disseminated MAC. The first possibility is that MAC was introduced directly into the tissue via direct inoculation, then disseminating to various organs over a period of time. Secondly, MAC is ubiquitous in the environment, including water sources, and can contaminate drinking water.(18) Water distribution systems have been reported as a possible source of infection in hospitals, homes, and commercial buildings.(1) Because MAC grows slowly, a six month lag between inoculation and clinical presentation, as seen in humans, is biologically plausible.(13) The third possibility is that the shrew had undiagnosed disseminated MAC at the time of arrival, and underwent a period of stress, which suppressed the immune system. Stress of any kind can tip the scale in the direction of the mycobacteria and produce fulminant infection. Systemic mycobacterial infection is an important differential diagnosis for an acute on-set of lameness in insectivores.
JPC Diagnosis:
Conference Comment:
In contrast to nontuberculous mycobacterial infections such as MAC, Mycobacterium tuberculosis is the prototypical etiologic agent resulting in granulomas and subsequent tubercle formation. Tubercles form when granulomas develop and coalesce. The center of a tubercle typically consist of white, friable material (caseous necrotic debris) derived from lysed cells and bound by epithelioid macrophages, multinucleated giant macrophages, lymphocytes, plasma cells, fibroblasts and collagen. With time, tubercles may become calcified.Â
Generally speaking, granulomas form as the result of cell-mediated immunity to a poorly degradable entity such as Mycobacteria. Initially, antigen presenting cells (APC), such as alveolar macrophages, present phagocytized mycobacterial antigen and MHC class II proteins to na+�-�ve T-cells. Binding of the T-cell receptor with mycobacterial antigen and exposure to the cytokine IL-12 released by the APC promotes differentiation from na+�-�ve CD4+ TH1 lymphocytes to activated T-helper 1 lymphocytes producing IL-2, which, in turn, activates additional T cells, tumor necrosis factor, and interferon-γ. Interferon-γ is an important factor in the differentiation of monocytes to epithelioid macrophages and multinucleated giant cells.(17)
References:
2. Appelberg R. Pathogenesis of mycobacterium avium infection: Typical responses to an atypical mycobacterium. Immunol. Res. 2006;35:179-190.Â
3. Biet F, Boschiroli ML, Thorel MF, et al. Zoonotic aspects of mycobacterium bovis and mycobacterium avium-intracellulare complex (MAC). Vet. Res. 2005;36:411-436.Â
4. Bruijnesteijn van Coppenraet LE, de Haas PE, Lindeboom JA, Kuijper EJ, et al. Lymphadenitis in children is caused by mycobacterium avium hominissuis and not related to 'bird tuberculosis'. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:293-299.Â
5. Cavanagh R, Begon M, Bennett M, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J. Clin. Microbiol. 2002;40:3281-3285.Â
6. Clarke KA, Fitzgerald SD, Zwick LS, et al. Experimental inoculation of meadow voles (microtus pennsylvanicus), house mice (mus musculus), and norway rats (rattus norvegicus) with mycobacterium bovis. J. Wildl. Dis. 2007;43:353-365.Â
7. Cvetnic Z, Spicic S, Benic M, et al. Mycobacterial infection of pigs in croatia. Acta Vet. Hung. 2007;55:1-9.Â
8. Desimone JA, Jr, Babinchak TJ, Kaulback KR, et al. Treatment of mycobacterium avium complex immune reconstitution disease in HIV-1-infected individuals. AIDS Patient Care STDS. 2003;17:617-622.Â
9. Durnez L, Eddyani M, Mgode GF, et al. First detection of mycobacteria in African rodents and insectivores, using stratified pool screening. Appl. Environ. Microbiol. 2008;74:768-773.Â
10. Fox LE, Kunkle GA, Homer BL, et al. Disseminated subcutaneous mycobacterium fortuitum infection in a dog. J. Am. Vet. Med. Assoc. 1995;206:53-55.Â
11. Gow AG, Gow DJ. Disseminated mycobacterium avium complex infection in a dog. Vet. Rec. 2008;162:594-595.Â
12. Hibiya K, Higa F, Tateyama M, et al. Mycobacteriosis as zoonotic disease--comparative pathological study on mycobacterium avium complex infection. Kekkaku. 2007;82:539-550.Â
13. Hoffman GS, Myers RL, Stark FR, et al. Septic arthritis associated with mycobacterium avium: A case report and literature review. J. Rheumatol. 1978;5:199-209.Â
14. Horn B, Forshaw D, Cousins D, et al. Disseminated mycobacterium avium infection in a dog with chronic diarrhoea. Aust. Vet. J. 2000;78:320-325.Â
15. Kasperbauer SH, Daley CL. Diagnosis and treatment of infections due to mycobacterium avium complex. Semin. Respir. Crit. Care. Med. 2008;29:569-576.Â
16. Koh WJ, Kim YH, Kwon OJ, et al. Surgical treatment of pulmonary diseases due to nontuberculous mycobacteria. J. Korean Med. Sci. 2008;23:397-401.Â
17. Kumar V, Abbas AK, Fausto N, et al. Cellular responses to stress and toxic insults: Adaptation, injury, and death. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:16,74.
18. Marinho A, Fernandes G, Carvalho T, et al. Nontuberculous mycobacteria in non-AIDS patients. Rev. Port. Pneumol. 2008;14:323-337.Â
19. Miller MA, Greene CE, Brix AE. Disseminated mycobacterium avium--intracellulare complex infection in a miniature schnauzer. J. Am. Anim. Hosp. Assoc. 1995;31:213-216.Â
20. Murdoch DM, McDonald JR. Mycobacterium avium-intracellulare cellulitis occurring with septic arthritis after joint injection: A case report. BMC Infect. Dis. 2007;7:9.Â
21. Naughton JF, Mealey KL, Wardrop KJ, et al. Systemic mycobacterium avium infection in a dog diagnosed by polymerase chain reaction analysis of buffy coat. J. Am. Anim. Hosp. Assoc. 2005;41:128-132.Â
22. Ogawa E, Murata M, Ohnishi H, et al. AIDS-related mycobacterium avium infection dissemination in a patient with endobronchial lesions associated with immune reconstitution inflammatory syndrome. Kansenshogaku Zasshi. 2008;82:341-346.Â
23. Oloya J, Opuda-Asibo J, Kazwala R, et al. Mycobacteria causing human cervical lymphadenitis in pastoral communities in the Karamoja region of Uganda. Epidemiol. Infect. 2008;136:636-643.Â
24. Rubin DS, Rahal JJ. Mycobacterium-avium complex. Infect. Dis. Clin. North Am. 1994;8:413-426.Â
25. Runyon EH. Whence mycobacteria and mycobacterioses? Ann. Intern. Med. 1971;75:467-468.Â
26. Steiger K, Ellenberger C, Schuppel KF, et al. Uncommon mycobacterial infections in domestic and zoo animals: Four cases with special emphasis on pathology. Dtsch. Tierarztl. Wochenschr. 2003;110:382-388.Â
27. Thorel MF, Huchzermeyer HF, Michel AL. Mycobacterium avium and mycobacterium intracellulare infection in mammals. Rev. Sci. Tech. 2001;20:204-218.Â
28. Tsang AY, Denner JC, Brennan PJ, et al. Clinical and epidemiological importance of typing of mycobacterium avium complex isolates. J. Clin. Microbiol. 1992;30:479-484.Â
29. Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am. Rev. Respir. Dis. 1979;119:107-159.Â
30. Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin. Infect. Dis. 1992;15:1-10.Â
31. Wong NM, Sun LK, Lau PY. Spinal infection caused by mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: A case report. J. Orthop. Surg. (Hong Kong). 2008;16:359-363.