Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 17
5 February, 2020
CASE II: WSC 2013 Case 1 (JPC 4032584).
Signalment: 119.2 kg (262.8 lb), 5-month-old, female, Hereford calf (Bos primmigenus)
History: The day prior to euthanasia, the calf was found struggling to walk, and within 4 hours was recumbent. Four days previously, another 6-month-old heifer calf was found dead on this farm.
Gross Pathology: Body condition was good with visible subcutaneous and visceral fat. There was mild interlobular septal edema in the lungs and a large amount of blood exuded from the cut surface. Few pinpoint to 3mm diameter white foci were present both on the surface and within the parenchyma of the liver. There was mild enlargement of the spleen (interpreted as a result of euthanasia). The serosa of the terminal segment of the ileum was reddened and the contents were watery and red. The urinary bladder contained a moderate amount of clear urine. Normally formed feces were present in the rectum.
Laboratory results: Immunofluorescence Test for Rabies Virus Antigen: Positive
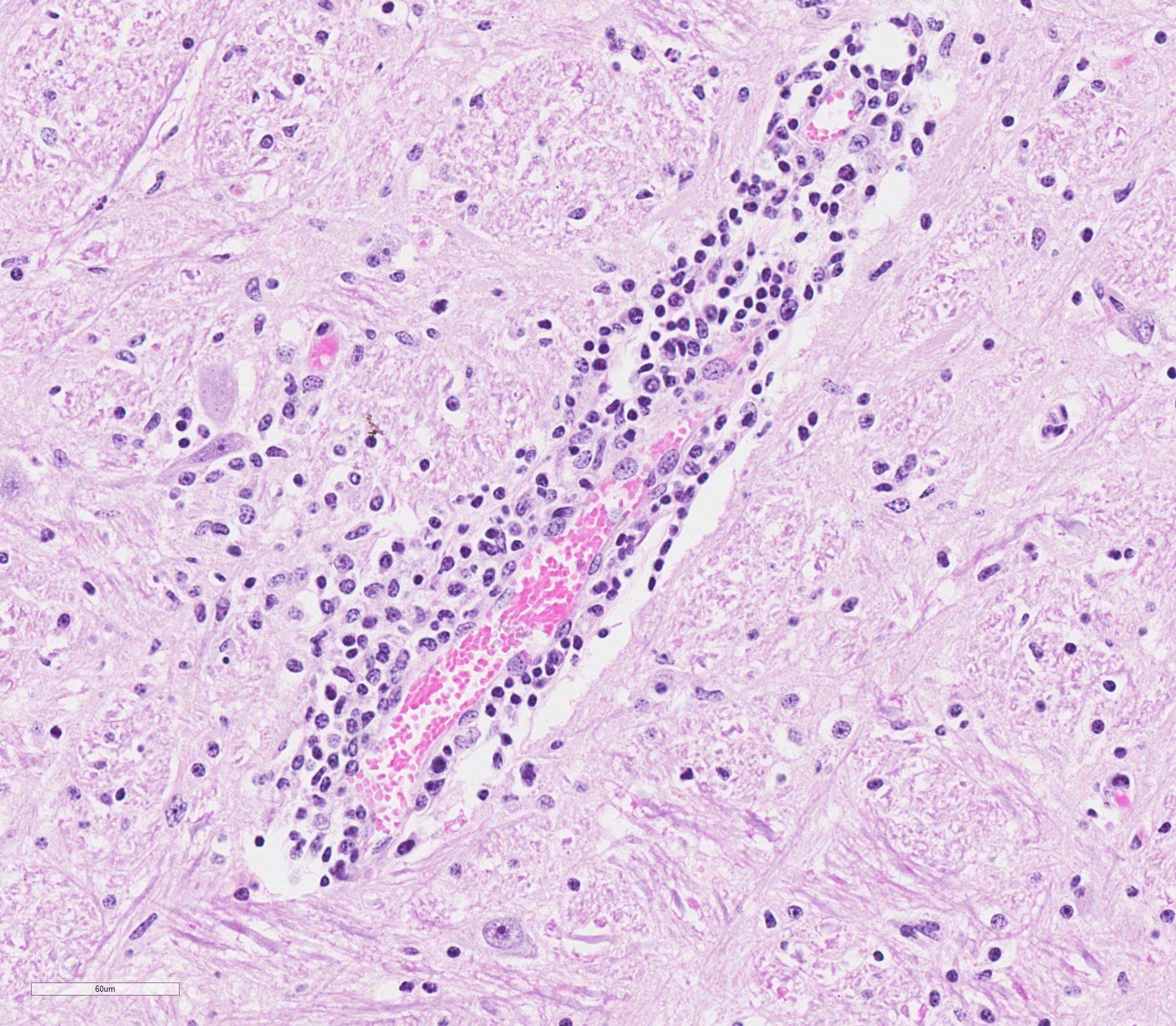
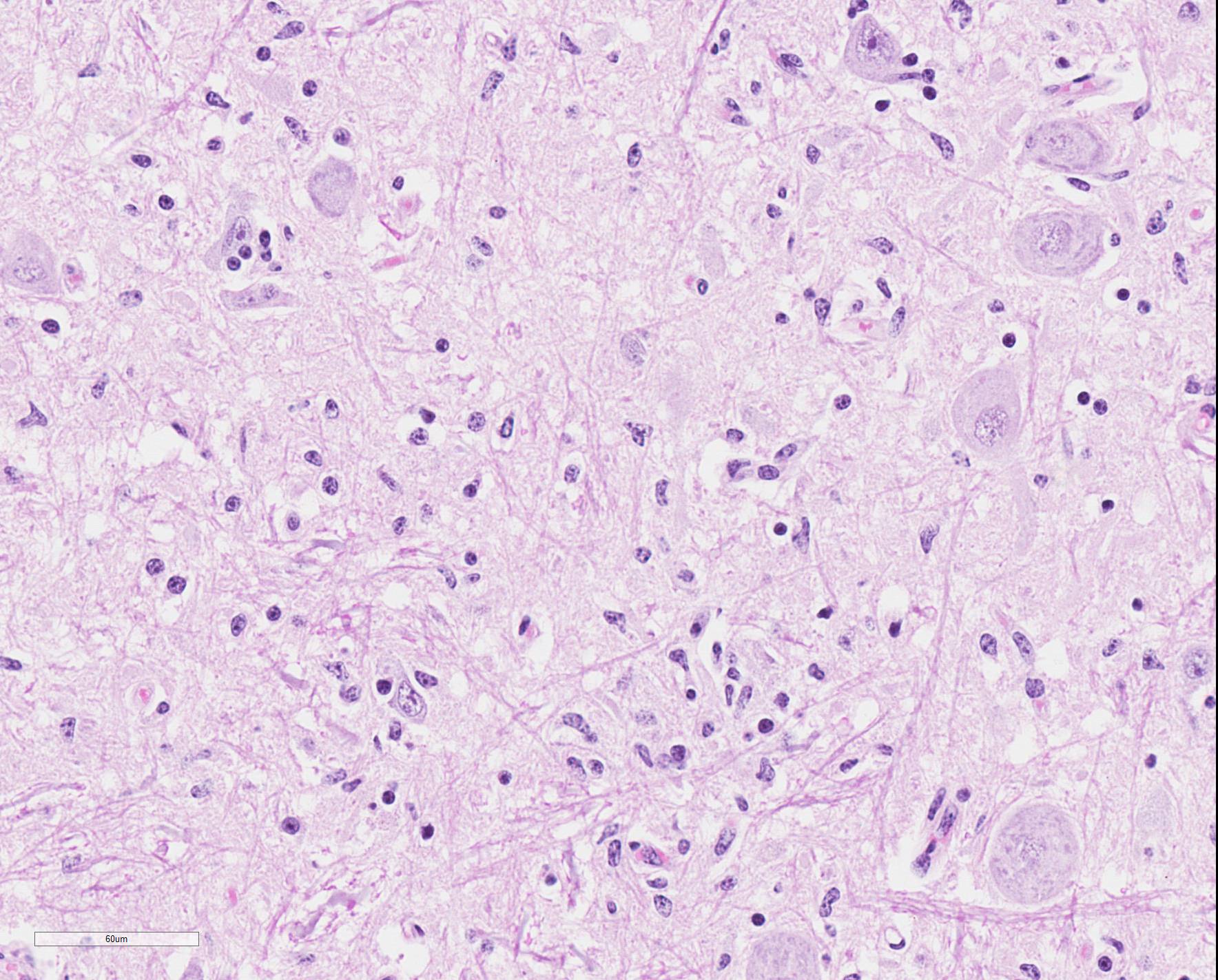
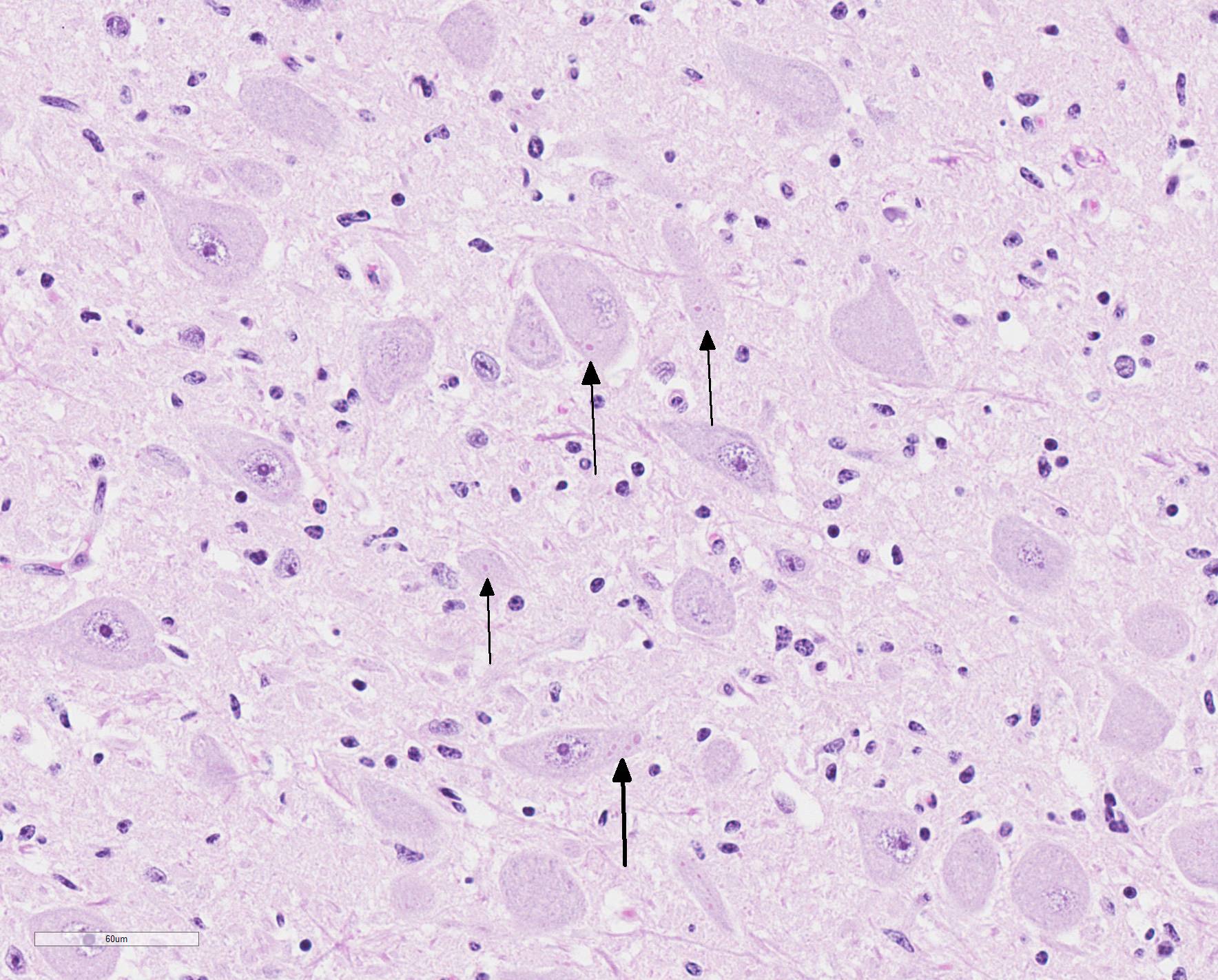
Microscopic Description: Medulla oblongata: Multifocally, blood vessels are surrounded by 1 to 6 cell thick cuffs of lymphocytes with lesser numbers of macrophages and plasma cells. There are occasional mild perivascular hemorrhages with edema. Increased numbers of glial cells, including astrocytes, microglia and oligodendrocytes, are present in the neuropil. Low numbers of neurons in areas of gliosis have one or multiple 2-4 um diameter, round to oval, eosinophilic, cytoplasmic inclusions (Negri bodies).
Contributor Morphologic Diagnosis:
Medulla oblongata: Encephalitis, lymphocytic, plasmacytic and histiocytic, multifocal, moderate, with gliosis and neuronal intracytoplasmic inclusions (Negri bodies), etiology consistent with rabies virus (RABV)
Contributor Comment: The microscopic and immunofluorescence findings in this calf indicate rabies encephalitis. Rabies virus (RABV) is a lyssavirus in the family Rhabdoviridae that causes acute, progressive encephalitis that is almost invariably fatal.6 Two biotypes of RABV exist, i.e. ?fixed? virus and ?street? virus.3 Fixed RABV is the stabilized laboratory biotype, which is highly neurotropic but does not create Negri bodies and is not secreted in saliva, while street RABV is the wild biotype that circulates in nature.3 All mammalian species can be infected by RABV, which is most commonly transmitted by bites of carnivores or bats. The virus can also enter via sensory nerve endings of the skin and mucous membranes, which has been observed in rare instances of aerosol exposure and ingestion (e.g. cannibalism and scavenging).5 RABV infection has been documented after human organ transplantation, e.g. cornea, kidney, pancreas and liver.5 The principal reservoir vector species within the USA and Canada vary by geographic region. Skunks, foxes and raccoons are important reservoir vectors in the continental United States and Canada, with raccoons of particular importance along the Atlantic coast, coyotes in southern Texas, and arctic foxes in Alaska.3,4 In central and South America, dogs are the most important reservoir vector, with rabies also reported in terrestrial wildlife, e.g. monkeys, wolves, skunks and foxes. The Indian mongoose (Herpestidae auropunctatus) has become an important vector species in the Caribbean after its introduction to control rodents.5 In Europe, the red fox is the principal reservoir vector, although dogs and the raccoon dog (Nyctereutes procyonoides) in Eastern Europe may serve as independent reservoir species or spill-over hosts dependent on the fox rabies cycle.5 In Africa and Asia, dogs are important reservoirs with spillover to other species a major problem, for example, rabies accounts for 25,000-30,000 human deaths annually in India8 and threatens the endangered Ethiopian wolf (Canis simensis) and the African wild dog (Lycaon pictus). Australia, New Zealand, Japan and the British Isles are free of RABV infection, but other lyssavirus species exist in bats in at least some of these countries.5 Fructivorous, insectivorous and vampire bats are capable of transmitting RABV.3,4
The bite of a rabid animal delivers the virus into the striated muscle and connective tissue at the site of inoculation. At the site of entry, the virus replicates for a short period in myocytes, then buds from the sarcolemma to invade the neuromuscular junction through conjugation with nicotinic acetylcholine receptors. Virus is then taken up at the axon terminus and delivered by retrograde axoplasmic flow within neurons to spinal cord and brain.3,4 Subsequent replication occurs in the limbic system and neocortex.3,4 The surface-exposed viral coat glycoprotein (RVG) is responsible for the neurotropism of RABV by binding to several neural tissue receptor molecules, specifically the neuronal cell adhesion molecule (NCAM) and the p75 neurotrophin receptor (p75NTR).3 In animal species that serve as vectors, the viral genome moves centrifugally from the central nervous system through peripheral nerves to a variety of tissues including the adrenal medulla, nasal mucosa, cornea, epidermis and most importantly, the salivary glands.1,3 In the salivary gland of these hosts, virions bud from plasma membranes at the apical (luminal) surface of mucous cells and into the intercellular canaliculi, and are released in high concentrations into the saliva resulting in highly infectious saliva.2,3,4
Following a 1-8 week incubation period, the clinical course of rabies is usually acute, lasting 1-2 days, but occasionally lasting up to 14 days. Following a prodromal phase, aberrant behavioral patterns occur in the infected animal. There are two recognized clinical forms of disease: furious and dumb or paralytic.3 Clinical features of the furious form include restlessness, nervousness, aggression, inability to swallow water, hypersalivation, exaggerated response to light and sound, and hyperesthesia. Following the furious phase, paralysis and recumbency ensues. Terminally, there are often convulsive seizures, coma and then respiratory arrest.2,3,4
Gross anatomical lesions specific to RABV infection are typically not evident. Histological lesions of rabies are those typical of nonsuppurative encephalomyelitis with ganglioneuritis. Inflammatory and degenerative changes are usually most severe from the pons to the hypothalamus and in the cervical spinal cord, with relative sparing of the medulla oblongata.3 The inflammatory reaction typically is one of perivascular cuffing and focal gliosis, although inflammatory changes may be minimal or absent in some cases.3 Intracytoplasmic viral inclusions, known as Negri bodies, are present most commonly in the hippocampus of carnivores and in cerebellar Purkinje cells of herbivores. Negri bodies are found in neurons that are otherwise histologically normal and are reported to be scarce in regions where inflammation is the most severe.3 The most severe lesions are commonly found in dogs, whereas other species (particularly ruminants, which are highly susceptible) may have minimal perivascular cuffing and few glial nodules in spite of having numerous Negri bodies.3 In ruminants, gliosis is prominent in both the white and gray matter. Perivascular cuffs are composed mostly of lymphocytes, and a ring of hemorrhage largely confined to the perivascular space is commonly found surrounding cuffed vessels.
Contributing Institution:
Department of Pathobiology and Veterinary Science
Connecticut Veterinary Medical Diagnostic Laboratory
University of Connecticut
www.patho.uconn.edu
JPC Diagnosis:
Brainstem: Meningoencephalitis, lymphocytic, diffuse, mild, with gliosis and occasional neuronal intracytoplasmic viral inclusions.
JPC Comment: As in Case 1 of this conference, a thorough and wide-ranging review by the contributor allows us to discuss some of the interesting aspects of the medical history of this well-known and global disease. The etymology of the word rabies itself is controversial, with authorites splet on whether it derived from the word rabias, meaning "rabies" in Latin or the Sanskrit terms rabhas or rabhasa.7
Rabies has likely been known to humans ever since the domestication of dogs,
estimated at up to 30,000 years ago. The first actual mention of rabies (and
penalties for owners of rabid dogs) were found in Sumerian tablets dating back
to 1930 BC - "If a dog becomes rabid [?] and the dog bites a man and causes his
death, the owner of the dog shall pay forty shekels of silver; if it bites a
slave and cuses his death, he shall pay fifteen shekels of silver". At this
time, herbs were often used as first attempts to cure the disease, and failing
that, incantations at the local temple. Dogs were thought to be more likely to
become rabid during a lunar eclipse, particularly if it occurred at the year&146;s
end. 7
Many cultures in antiquity made attempts to prevent the transmission of disease. In Persia, cutting off a dogs tail when they are 40 days old was done to protect them from the bite of another rabid animals. One prevention mistep in China during the Jin dynasty was to treat by applying the biting dog&146;s brain tissue to the bite wound to prevent disease. Pedianus Doscorides, a Roman physician living in Turkey may have started down a more appropriate path, calling for the heat cauterization of bites by rabid dogs, but this was later denounced by other Roman doctors as unnecessary brutal. 7
In ancient Greece, many medical texts showed an increasing awareness of rabies and its effects, with detailed recunts of its symptoms in man and animals, recognition of a latency period between the bite and onset of symptoms, and even palliative care. 7
The
Middle Ages showed an advance in the diagnosis of clinical rabies with
hydrophobia described in affected humans (and noted to be absent in dogs),
paralyic rabies, and the need for thorough cleansing of bite wounds as a
potential treatment (although the agent was still considered to be a toxin).
Religion impacted early vaccination, with dogs vaccinated with a white-hot "Key
of Saint Hubert" to prevent the contraction of rabies, and eventually was
applied to the wounds of people bitten by dogs believed to be rabid. Alternatively,
an incision would be made in the unfortunate bite victim, and threads from the
Saint?s cassock were implanted, accompanied by prayers and fasting. Patients
not responding to these surefire cures were unfortunately suppressed between
mattresses (an alternative form of treatment in Europe until the nineteenth
century. 7
The Renaissance saw rabies as a major entry in medical texts of the time, with
an entire volume written on it by Dr. Julien Le Paulmier. The practices at Saint-Hubert
were condemned, and cleansing the wound thoroughly was considered a beneficial
practice among clinicians. As the saliva of infected dogs was now being looked
at as the potential vector for transmission of the disease, an enterprising
Prussian artillery general Kazimierz Siemienowicz tried filling hollow
artillery shells with the saliva of rabid dogs and firing them at the enemy in
the mid 1600&146;s. 7
The exploration of the new world brought dog-associated rabies to a continent which had largely recognized only bat-associated rabies, and spread to local wildlife such as mongooses in the Caribbean islands. 7
In
Renaissance Europe, the perception of the pathogenesis was evolving. The
current thought was that rabies was transmitted by "seeds" (semina) in
the saliva, and that transmission after a bite wound was an inconstant event.
The first 15-day quaratine period was proposed by Joseph-Ignace Guillotin (yes,
that Guillotin) in 1776. Conversely, he also suggested inoculation experiments
on prisoners awating capital punishment (which was supported by Louis Pasteur
years later), but thankfully this practice did not occur. Advances in
treatment continued to lag behind, with application of the "hair of the dog" to
the wound, and even eating omelets flavored with "dog-rose root" (previoulsy
suggested over a fifteen hundred years before) as examples of treatments of the
time. 7
In the early 1800?s, the newly minted "scientific approach" made great strides
on elucidating the transmission of rabies and potential prevention. In
1813-1814, several researchers in Europe clearly transmitted the disease with
the saliva of rabid dogs humans, and sciatic nerve tissue from a rabid cat.
Francois Magendie, 30 years later, first suggested the agent was not a poison
but a virus, and in 1879, Paul-Henri Duboue first proposed the theory of
neuronal transmission. 7
It
was in this time of significant advance that Louis Pasteur was developing a
live attenuated vaccine developed by a dessication technique and successfully
used on dogs subsequently challenge with live rabies virus. The first human
trial, tried on a child who was exhibiting sign of rabies, was met with
failure, but a second trial, on 9-year-old Joseph Meister, only two days after
the bite of a rabid dog and before the demonstration of any clinical signs was
a success. Over 11 days, a total of 13 injections with initially attenuated and
progressively more virulent virus allowed Master Meister to develop immunity
against the virus and survive. A second successful attempt three months later
in another child proved the efficacy of post-exposure prophylaxis, and since
that time, PEP has saved countless lives around the world. In 1932, the League
of Nations published records of over 115,000 patients that had received PEP,
with a combined rabies mortality of oly 0.4%).7
Research on the virus itself proceeds apace during this time. In 1903,
Adelphi Negri and Lina Luzzani-Negri desibed the fist RABV-neuronal interaction
and the cytoplasmic inclusions that still bear their names. The virus itself
was first visualized ultrastructually in the early 1960s, and its viral genome
was mapped in 1988. These and many other advances allowed modification and
shortening of PEP protocols, and laid the groundwork for marked advances in
vacine research. 7
However, these advances have only transformed rabies from a globally prevalent
scourge, to a global neglected disease, following its eradication in North
America and Europe. As of 2017, na estimated 59,000 people die each year of
rabies, easily eclipsing similar deaths from Ebola outbreaks. Current attempts
to curb these numbers include significant vaccination efforts in animal
species, and human rabies vaccination in endemic areas. 7
References:
1. Balachandr A, Charlton K. Experimental rabies infection of non-nervous tissues in
skunks (Mephitis mephitis)
and foxes (Vulpes vulpes). Vet Pathol. 1994;31:93-102.
2. King AA, Turner GS. Rabies: a
review. J Comp Path. 1993;108:1-39.
3. Maxie MG, Youssef S.
Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmer?s, Pathology of
Domestic Animals, vol. 1. 5th ed. Edinburgh, UK: Elsevier
Limited; 2007:413-416.
4. Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Veterinary
Virology, 3rd ed. San Diego, CA: Academic Press; 1999:432-439.
5. Nel LH, Markotter W. Lyssaviruses. Crit Rev Microbiol.
2007;33:301-324.
6. Stein LT, Rech RR, Harrison L, Brown CC. Immunohistochemical study of rabies
virus within the central nervous system of domestic and wildlife species. Vet Pathol. 2010;47:630-633.
7. Tarantola A. Four thousand years of concepts relating
to rabies in animals and humans, its prevention and its cure. Trop Med Inf
Disease 2017; 2(2). Pil E5. Doi: 10.3390/tropicalmed202005.
8. Wunner WH, Briggs, DJ. Rabies in the 21st century. PLoS Negl Trop Dis. 2010;4(3):e591.