Signalment:
Gross Description:
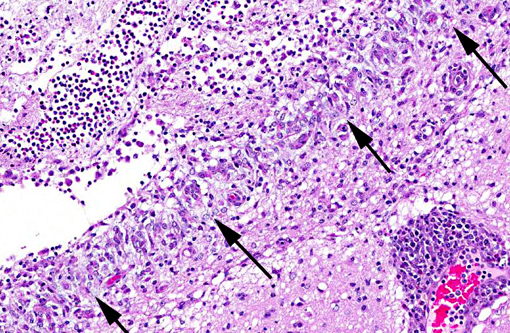
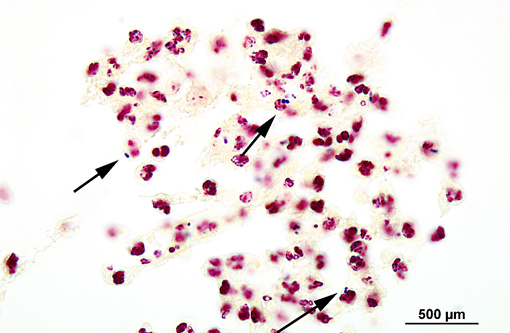
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Streptococcus suis is a gram-positive, facultative anaerobic, α-hemolytic streptococcus belonging to Lancefield group D.(3,11) More than 30 serotypes have been identified, and most infections in pigs in most countries are caused by serotype 2.(3) Disease is mainly seen in weanlings and growing pigs, with incidence peaking at weaning, and may include septicemia, serositis, meningitis, polyarthritis, pneumonia, abortions, abscesses and endocarditis.(3,11)
Outbreaks of S. suis generally have low morbidity and mortality ranging from 0-20%, depending on treatment.(11) Carriers are significant factors in disease transmission, and outbreaks may occur in closed herds.(11) Stress can predispose to infection, and concurrent infections increase morbidity.(11)
JPC Diagnosis:
Conference Comment:
There are several Streptococcus species of veterinary importance in addition to S. suis. S. canis infection in neonatal and adult dogs (and less commonly cats) has been associated with pneumonia, abortion, septicemia, endocarditis, necrotizing fasciitis, keratitis, lower urinary tract infections, cholangiohepatitis, prostatic abscesses, mastitis, arthritis and meningoencephalitis.(5) S. equi subsp. equi causes equine strangles, a contagious infection of the upper respiratory tract and local lymph nodes;(12) it has also been linked with immune mediated vasculitis and purpura hemorrhagic a.(7) S. equi subs. zooepidemicus and S. equisimilis are associated with equine reproductive disease, but have also been isolated from the lung, liver, brain, kidney and joints.(8,12) S. equi subs. zooepidemicus also causes bursitis or fistulous withers in horses,(7) and was implicated in an outbreak of acute hemorrhagic pneumonia in more than 1,000 shelter dogs in California.(2) S. agalactiae (and less commonly S. dysgalactiae and S. uberis) are important causes of bovine mastitis.(9) S. iniae is a significant aquatic pathogen of tilapia and other reef fish, which causes necrosis, inflammation and vasculitis.(4) Furthermore, several species of Streptococcus are zoonotic, including S. can is,(5) S. equip sub. zooepidemicus,(12) S. iniae(4) and S. suis.(11,14)
Conference participants outlined several potential causes for the gross and histologic lesions associated with S. suis in swine. The fibrinous polyserositis often noted grossly at necropsy(11) could also occur secondary to Hemophilus parasuis or Mycoplasma hyorhinus infection.(1) Ruleouts for the microscopic lesions of meningoencephalitis and ventriculitis include salt toxicity, edema disease and postweaning multisystemic wasting syndrome (PMWS). Salt toxicity is characterized by cortical laminar necrosis/malacia with eosinophilic meningoencephalitis.(7) Shiga-toxin producing E. coli (STEC), the etiologic agent of porcine edema disease, induces fibrinoid vascular change and necrotizing vasculitis with subsequent edema in various tissues, including the brain.(6) Cerebellar spongiosis, necrotizing vasculitis, edema and hemorrhage are occasionally described in conjunction with porcine circovirus type 2 and PMWS.(10) However, as noted by the contributor, the ventricular localization and fibrinosuppurative character of the lesions in this case are fairly specific for S. suis.(11)
References:
2. Erol E, Locke SJ, Donahe JK, Mackin MA, Carter CN. Beta-hemolytic Streptococcus spp. from horses: a retrospective study (2000-2010). J Vet Diagn Invest. 2012;24(1):142-147.
3. Kahn CM, ed. Streptococcus suis infection. In: The Merck Veterinary Manual, 9th ed. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Retrieved from http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/54302.htm
4. Keirstead ND, Brake JW, Griffin MJ, Halliday-Simmonds I, Thrall MA, Soto E. Fatal septicemia caused by the zoonotic bacterium Streptococcus iniae during an outbreak in Caribbean reef fish. Vet Pathol. 2013 Sep 27. [Epub ahead of print]. Accessed 19 October 2013.
5. Lamm CG, Ferguson AC, Lehenbauer TW, Love BC. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet Pathol. 2010;47(3):387-395.
6. Matise I, Sirinarumitr T, Bosworth T, Moon HW. Vascular ultrastructure and DNA fragmentation in swine infected with Shiga toxin-producing Escherichia coli. Vet Pathol. 2000;37(4):318-327.
7. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:173, 257, 358.
8. Pesavento PA, Hurley KF, Bannasch MJ, ARtiushin S, Timoney JF. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet Pathol. 2008;45(1):51-53.
9. Schlafer DH, Miller RB. Female Genital System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed, Vol. 3. Philadelphia, PA: Elsevier; 2007: 552-554.
10. Seelinger FA, Brugmann ML, Greiser-Wilke I, Verspohl J, Segales J, et al. Porcine circovirus type 2-associated cerebellar vasculitis in postweaning multisystemic wasting syndrome (PMWS)-affected pigs. Vet Pathol. 2007;44(5):621-634.
11. Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Comm.1997;21:381-407.
12. Timoney, JF. The pathogenic equine streptococci. Vet Res. 2004;35(4):397-409.
13. Vasconcelos D, Middleton DM, Chirino-Trejo JM. Lesions caused by natural infection with Streptococcus suis type 9 in weaned pigs. J Vet Diagn Invest. 1994;6:335-341.
14. Zheng P, Zhao YX, Zhang AD, Kang C, Chen HC, et al. Pathologic analysis of the brain from Streptococcus suis type 2 experimentally infected pigs. Vet Pathol. 2009;46(3):531-535.