Signalment:
Gross Description:
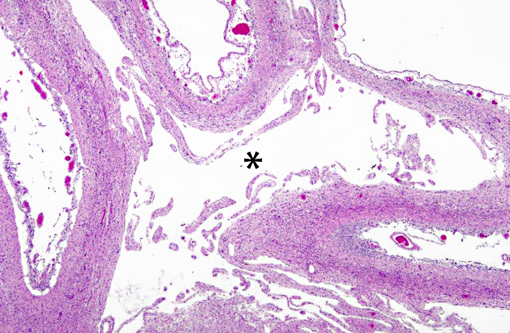
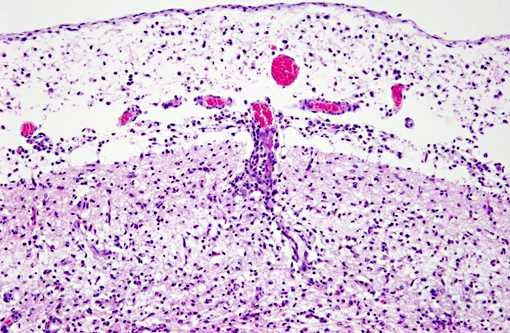
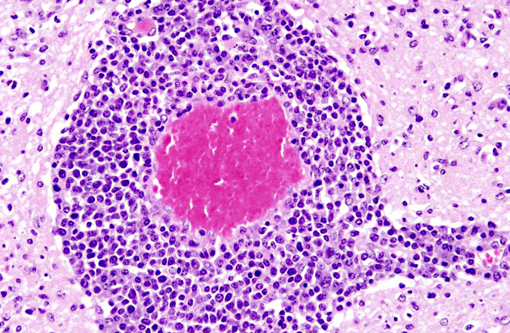
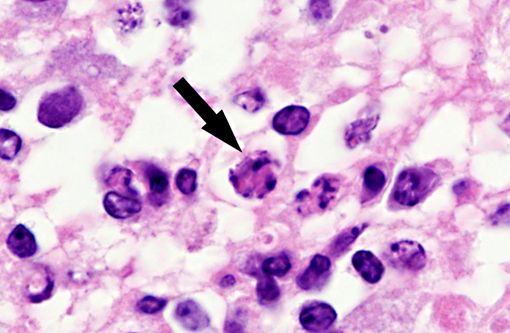
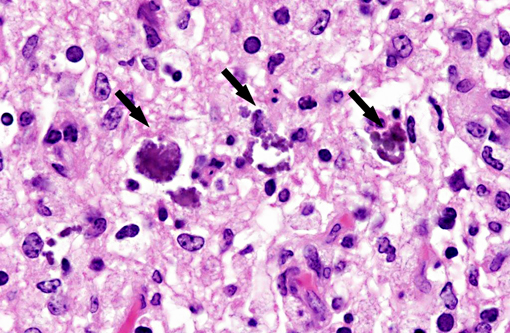
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Based on metagenomic analysis,(11) SBV belongs to a group of teratogenic viruses causing arthrogryposis and hydranencephaly (AG/HE). These malformations in cattle caused by Akabane virus represent an entity called enzootic bovine arthrogryposis and hydranencephaly.(20)
Until now the pathogenesis of SBV infection is not fully understood. Due to a genetic relationship to other viruses of the genus Orthobunyavirus, a similar pathogenesis is suggested.(12) Furthermore, the SBV-induced pathology cannot be differentiated from infections with Akabane virus(12) which requires an identification at the molecular level with PCR.(11) Appropriate samples for the detection of viral nucleotides by means of this technique are external placental fluid, umbilical cord, cerebrum, and spinal cord.(2) Placental fluid and umbilical cord samples can be taken easily without necropsy. The central nervous system is accessible in animals submitted for necropsy without placental fluid and only umbilical cord remnants.(2) Spleen, cartilage, placental fluid from the stomach, and meconium rarely revealed a positive PCR result in SBV-infected animals and therefore these samples should be avoided for virus detection.(2) In addition, placenta and placental fluid of SBV-infected animals contain huge amounts of virus.(2) So far the host range of SBV seems to be restricted to ruminants and there is no evidence of a zoonotic risk.(6)
A recent study compared genomic RNA of SBV with Sathuperi and Shamonda viruses indicating that all viruses belong the genus Orthobunyavirus and that SBV originates from a re-assortment of Sathuperi and Shamonda virus.(26)
In general, climate change is suggested to be the most important factor for the occurrence of arthropod-borne virus-infections in Europe.(9) SBV is supposed to be transmitted by arthropods, but the high numbers of initial SBV infections in Europe suggest additional routes of transmission, e.g. direct contact, fecal-oral route or aerosols.(1)
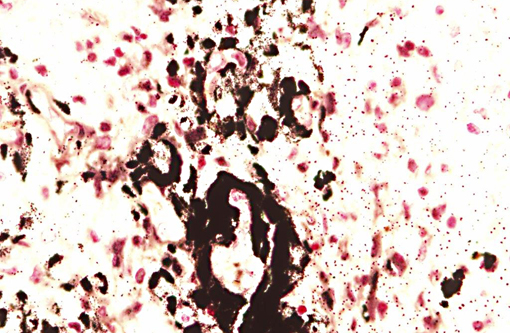
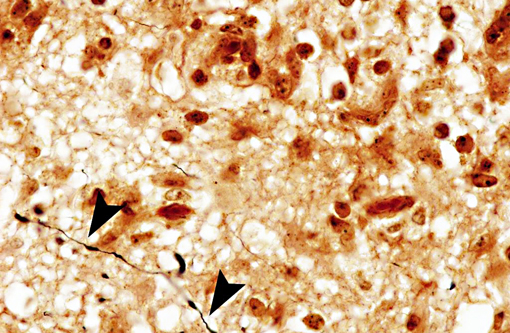
Interestingly, in the present case the lambs CNS showed liquefactive necrosis with porencephaly and additional non-suppurative inflammation. Studies with the closely related Akabane virus showed that the virus crosses the placenta after viremia and replicates in fetal cells of the central nervous system. The virus prefers rapidly dividing cells and causes damage to neurons.(24) Depending on the time point of infection, CNS pathology varies. According to Akabane virus infection, it is suggested that transplacental SBV infection at early stages of gestation causes severe brain lesions, like hydranencephaly due to a widespread loss of neuronal tissue. The severity of brain damage is less extensive, if the infection occurs to a later time point of gestation. Infection during the 2nd trimester may result in a more focal necrosis of the CNS characterized by porencephaly. This porencephaly can be associated with a meningoencephalitis.(20) Assuming that Akabane virus and SBV have a similar pathogenesis, an infection during the 2nd trimester is suggested in the present case. Our study revealed that only 20% (12 out of 58 animals) of all RTqPCR positive tested CNS samples of ruminants (lambs, goat kids and calves) showed meningoencephalitis with or without CNS malformations.(10) Infiltration of lymphocytes and macrophages showed a perivascular pattern. Furthermore, gitter cell formation and neuronal necrosis were present. For further characterization of the lesions several special stains were applied including von Kossa-�s stain, Luxol fast-blue stain, and Bielschowsky-�s silver impregnation. Intralesional amorphous basophilic material stained positive with a von Kossa stain indicating intralesional mineralization, which was found multifocally in the thin cortical areas of the pore. Demyelination was shown as reduced staining intensity in the cortex containing pores. Bielschowsky-�s silver impregnation was used to investigate the extent of axonal alterations. The amount of positive axons was reduced.Â
The following table lists possible etiological differentials of SBV infection. The described histopathological lesions are not characteristic for a particular virus. The etiology has to be proven with other methods, e.g. molecular techniques, like PCR. Further epidemiological information as well as the geographical area (e.g. Asia, Europe, USA) of occurrence have to be considered. The table summarizes information of naturally infected animals; results of experimentally infected animals were not included.
Table 1: Overview on virus infections, their pathology in offspring after natural transmission, susceptible species and the transmission mode representing possible etiological differentials of Schmallenberg virus infection.
| Virus | Disease name | Gross findings | Histology | Species | Transmission |
| Schmallenberg virus(4,10,11)Orthobunyavirus, Bunyaviridae | Arthrogryposis, vertebral malformations, brachygnathia inferior, hydran- and porencephaly, internal hydrocephalus, cerebellar hypoplasia, micromyelia | Lymphohistiocytic meningoen-cephalomyelitis, CNS malacia, gliosis, muscular hypoplasia | Ruminants: sheep, goats, cattle, bisons | Arbovirus: mosquitos and midges: e. g. Culex obsoletus, Culex dewulfi |
|
| Akabane virus(3,13,15,16,18,22) Orthobunyavirus, Bunyaviridae | Enzootic bovine arthrogryposis and hydranencephaly; congenital arthrogryposis-hydranencephaly syndrome (CAHS) | Arthrogryposis, vertebral malformations, hydranencephaly, CNS cyst formation | Non-suppurative encephalo-myelitis, neuronal loss in the spinal cord, muscular dysplasia | Herbivores: cattle, horses, donkeys, sheep, goats, camels, buffaloes, pigs | Arbovirus: mosquitos and midges, eg. Culex sp., Aedes sp., Culicoides imicola |
| Bovine virus diarrhea virus(20) Pestivirus, Flaviviridae | Cerebellar hypoplasia, mummification, runting, microencephaly, hydranencephaly, hydrocephalus, microphthalmia, cataract, brachygnathism, thymic aplasia, hypotrichosis, alopecia, pulmonary hypoplasia | perivascular non-suppurative meningeal infiltration, CNS malacia, hypomyelinogenesis, retinal degeneration, optic neuritis, myocarditis | Cattle, sheep, goat, pig | Excretions, inhalation, ingestion, semen, contaminated embryo transfer fluid | |
| Blue tongue virus(19,20) Orbivirus, Reoviridae | Porencephaly, hydranencephaly, hydrocephalus, subcortical cysts, cerebellar dysgenesis, runting | Necrotizing meningoencephalitis, interstitial pneumonia, mono-nuclear cells in kidney and liver | Sheep, wild ruminants, camelids, cattle, zoo carnivores(14) | Arbovirus: Culicoides sp. | |
| Border disease virus(20) Pestivirus, Flaviviridae | Border disease Condition: hairy shaker | Embryonic death, mummification, fleece abnomalities, runting, cerebellar hypo- and dysplasia, micro-, por- and hydranencephaly, leukoencephalomalacia, micro-myelia, starvation, cardiac abnormalities, rarely: arthrogryposis, kyphoscoliosis | Dys- and hypomyelination | Sheep, goats, pigs, cattle | Oral, conjuctival, intranasal, genital, semen |
| Cache valley virus, syn. Bunyamwera virus(5,20) Orthobunyavirus, Bunyaviridae | Arthrogryposis, hydrocephalus, hydranencaphaly, microcephaly, vertebral malformations, cerebellar hypoplasia, micromelia, porencephaly | Necrosis and loss of neuropil and motor neurons, myositis, poorly developed myocytes | Sheep, deer, caribou, pigs, horses, cattle, raccoons, foxes, man | Arbovirus: Culicoides sp.,Culiseta sp., Anopheles sp. | |
| Chuzan virus, syn. Palyamvirus(20) Orbivirus, Reoviridae | Hydranencephaly, cerebellar hypoplasia, hydrocephalus, microcephaly | Cattle | Arbovirus: Culicoides oxystoma | ||
| Classical swine fever virus(20) Pestivirus, Flaviviridae | Hog cholera | Mummification, runting, stillbirth, pulmonary hypoplasia, pulmonary artery malformation, micrognathia, arthrogryposis, cerebellar hypoplasia, microcephaly, defective myelination | Necrotizing vasculitis in CNS, intestine, skin, lymphoid depletion, endothelial degeneration, valvular fibrosis, portal fibrosis (liver), interstitial pneumonia, neuronal degeneration | Pigs, cattle, sheep, goats | Excretions, ingestion |
| Rift valley fever virus(20,23) Phlebovirus, Bunyaviridae | Intra-uterine fetal death | Hepatic necrosis, acidophilic intranuclear inclusions, cholestasis, degeneration of lympho-cytes, heart muscle and renal tubules | Sheep, cattle, goats, man | Arbovirus: Aedes sp., Culex sp. | |
| Wesselsbron virus(20) Flavivirus, Flaviviridae | Mummification, hydranencephaly, arthrogryposis, hydrops amnii, porencephaly, cerebellar hypoplasia | Non-suppurative meningo-encephalomyelitis, eosinophilic, intranuclear inclusions, liver: degeneration, necrosis, Kupffer cell hyperplasia, hepatitis, hyper- and inflammation of bile ducts | Sheep, cattle, man | Arbovirus | |
| Aino virus, syn. Shuni virus(3,21,25) Orthobunyavirus, Bunyaviridae | Arthrogryposis-hydranencephaly syndrome (CAHS) | Hydranencephaly, arthrogryposis, cerebellar hypoplasia | Necrotizing encephalopathy, perivascular cuffing with lymphoid cells, neuronal mineralization | Cattle, sheep | Arbovirus: Culicoides brevitarsis, Culex tritaeniorhynchus |
JPC Diagnosis:
Conference Comment:
References:
2. Bilk S, Schulze C, Fischer M, Beer M, Hlinak A, Hoffmann B. Organ distribution of Schmallenberg virus RNA in malformed newborns. Veterinary Microbiology. 2012:159:236-8.
3. Brenner J, Tsuda T, Yadin H, Chai D, Stram Y, Kato T. Serological and clinical evidence of a teratogenic Simbu serogroup virus infection of cattle in Israel, 2001-2003. Vet Ital. 2004;40:119-123.
4. Conraths F, Beer M, Peters M. "Schmallenberg-Virus": Eine neue Infektionskrankheit bei Wiederk+�-�uern. Tier+�-�rztliche Umschau. 2012;5:147-150.
5. Edwards JF, Livingston CW, Chung SI, Collisson EC. Ovine arthrogryposis and central nervous system malformations associated with in utero Cache Valley virus infection: spontaneous disease. Veterinary Pathology. 1989;26:33-39.
6. Eurosurveillance editorial team: European Food Safety Authority publishes its second report on the Schmallenberg virus. Euro Surveillance. 2012;17: pii=20140.
7. Garigliany MM, Hoffmann B, Dive M, Sartelet A, Bayrou C, Cassart D, et al. Schmallenberg virus in calf born at term with porencephaly, belgium. Emerging Infectious Diseases. 2012;18:1005-1006.
8. Gibbens N. Schmallenberg virus: a novel viral disease in northern Europe. Vet Rec. 2012;170:58.
9. Gould EA, Higgs S, Buckley A, Gritsun TS. Potential arbovirus emergence and implications for the United Kingdom. Emerging Infectious Diseases. 2006;12:549-555.
10. Herder V, Wohlsein P, Peters M, Hansmann F, Baumg+�-�rtner W. Salient lesions in domestic ruminants infected with the emerging so-called Schmallenberg Virus in Germany. Veterinary Pathology. 2012;49(4):588-91.
11. Hoffmann B, Scheuch M, H+�-�per D, Jungblut R, Holsteg M, Schirrmeier H, et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerging Infectious Diseases. 2012;18:469-472.
12. H+�-�per D, Wernike K, Eschbaumer M, Conraths F, Hoffmann B, Schirrmeier H, et al. "Schmallenberg-Virus" - Ein neues Virus in Europa. Deutsches Tier+�-�rzteblatt. 2012;4:500-505.
13. Huang CC, Huang TS, Deng MC, Jong MH, Lin SY. Natural infections of pigs with akabane virus. Veterinary Microbiology. 2003;94:1-11.
14. Jauniaux TP, De Clercq KE, Cassart DE, Kennedy S, Vandenbussche FE, Vandemeulebroucke EL, et al. Bluetongue in Eurasian lynx. Emerging Infectious Diseases. 2008;14:1496-1498.
15. Konno S, Moriwaki M, Nakagawa M. Akabane disease in cattle: congenital abnormalities caused by viral infection. Spontaneous disease. Veterinary Pathology. 1982;19:246-266.
16. Kono R, Hirata M, Kaji M, Goto Y, Ikeda S, Yanase T, et al. Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet Res. 2008;4:20.
17. Kupferschmidt K. Infectious disease. Scientists rush to find clues on new animal virus. Science. 2012;335:1028-1029.
18. Lee JK, Park JS, Choi JH, Park BK, Lee BC, Hwang WS, et al. Encephalomyelitis associated with akabane virus infection in adult cows. Veterinary Pathology. 2002;39:269-273.
19. Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. Journal of Comparative Pathology. 2009;141:1-16.
20. Maxie M, Youssef S. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Vol. 1-3. Philadelphia, PA: Saunders Elsevier; 2007.
21. Noda Y, Uchinuno Y, Shirakawa H, Nagasue S, Nagano N, Ohe R, et al. Aino virus antigen in brain lesions of a naturally aborted bovine fetus. Veterinary Pathology. 1998;35:409-411.
22. Parsonson IM, McPhee DA, Della-Porta AJ, McClure S, McCullagh P. Transmission of Akabane virus from the ewe to the early fetus (32 to 53 days). Journal of Comparative Pathology. 1988;99:215-227.
23. Rippy MK, Topper MJ, Mebus CA, Morrill JC. Rift Valley fever virus-induced encephalomyelitis and hepatitis in calves. Veterinary Pathology. 1992;29:495-502.
24. St. George T, Kirkland P. Diseases caused by Akabane and related Simbu-group viruses. In: Coetzer J, Tustin R, eds. Infectious Diseases of Livestock. 2nd edition. eds. 2nd ed. Oxford, UK: Oxford University Press; 2004:1029-1036.
25. Uchinuno Y, Noda Y, Ishibashi K, Nagasue S, Shirakawa H, Nagano M, et al. Isolation of Aino virus from an aborted bovine fetus. Journal of Veterinary Medical Science. 1998;60:1139-1140.
26. Yanase T, Kato T, Aizawa M, Shuto Y, Shirafuji H, Yamakawa MT. Genetic reassortment between Sathuperi and Shamonda viruses of the genus Orthobunyavirus in nature: implications for their genetic relationship to Schmallenberg virus. Archives of Virology, 2012;157(8):1611-6.