Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
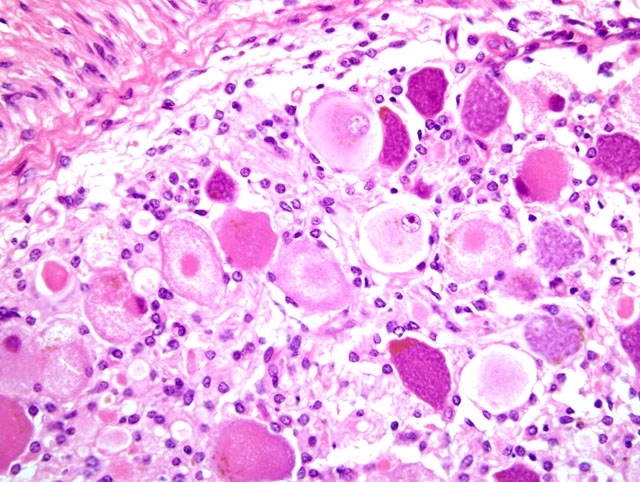
In acute cases, which are more common, at post-mortem examination there is typically distension of the stomach, and there may be evidence of gastro-esophageal reflux, i.e. distal esophageal ulceration. The large colon is usually filled with firm fecal balls that have a black coating of blood products. Histological lesions are classically described as being found in postganglionic sympathetic and parasympathetic neurons; typically at post-mortem the coeliacomesenteric and cranial cervical ganglia are sampled in addition to intestinal tract specimens. Normal neuronal numbers in these sites have been published.(6) Lesions are also found in parasympathetic terminal cardiac ganglia and have been associated with a functional reduction in cardiac autonomic control.(5) Cytoplasmic vacuolation, chromatolysis and necrosis of neurons is noted, and numbers of neurons are reduced significantly.(1) More chromatolytic neurons are noted in acute than in chronic cases. However, lesions have also been reported in general somatic efferent and general visceral efferent lower motor neurons in the brainstem and spinal cord including chromatolysis of lower motor neurons of the general visceral efferent nucleus of cranial nerves III and X, and the general somatic efferent nuclei of cranial nerves III, V, VII and XII.(2) For antemortem diagnosis it is typical to submit a full-thickness biopsy specimen from the ileum as pathology in the intestinal tract is seen most consistently and severely in that segment, particularly in the submucosal plexuses.(1)
The cause is not known, but the most popular current theory being, with some circumstantial evidence, that this is a form of botulism (Clostridium botulinum type C).(4)
JPC Diagnosis:
Conference Comment:
Conference participants based a discussion of chromatolysis on the example provided here. Chromatolysis is a histologically appreciable change in the soma resulting from dispersal of Nissl granules, which are composed of rough endoplasmic reticulum; chromatolysis may be classified as central or peripheral based on its location within the soma and is best demonstrated with special stains, such as cresyl violet. Cells undergoing central chromatolysis are usually swollen with a distinctly eccentric nucleus, while peripheral chromatolysis is more commonly associated with cell shrinkange. Chromatolysis is always considered to be a lesion; however, correctly identifying and interpreting it is predicated on knowing the normal distribution of Nissl granules at a given location.(3) In the autonomic ganglia, for instance, Nissl granules are normally concentrated at the periphery of the soma, so chromatolysis results in intense central eosinophilia, often with multiple fine clear vacuoles at the periphery of the soma, as is superbly demonstrated in this case.(7) Chromatolysis is a nonspecific reaction that essentially represents a metabolic adaptation to change. For instance, in cases of axonal injury, chromatolysis is evidence of the anabolic response required for regeneration, and has in this context been referred to as the axon reaction. When axonal regeneration is complete, the chromatolytic soma may pass through a densely basophilic phase before returning to normal. Alternatively, chromatolysis may precede cell death, the likelihood of which increases with increasing proximity of the axonal lesion to the cell body. Chromatolysis is also a characteristic feature of numerous motor neurodegenerative conditions and perinatal copper deficiency in sheep and goats.(3)
The dysautonomias of horses, cats, dogs, and hares share not only compelling epidemiological and geographical commonalities, but also a similar distribution of central neuropathology, lending further credence to the suggestion of a common etiology.(2)
References:
2. Hahn CN, Mayhew IG, de Lahunta A: Central neuropathology of equine grass sickness. Acta Neuropathol 102:153-159, 2001
3. Maxie MG, Youssef S: Nervous system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, p. 444. Elsevier Saunders, Philadelphia, PA, 2007
4. Newton JR, Hedderson EJ, Adams VJ, McGorum BC, Proudman CJ, Wood JLN: An epidemiological study of risk factors associated with the recurrence of grass sickness (dysautonomia) on previously affected premises. Equine Vet J 36:105-112, 2004
5. Perkins JD, Bowen IM, Else RW, Marr CM, Mayhew IG: Functional and histopathological evidence of cardiac parasympathetic dysautonomia in equine grass sickness. Vet Rec 146:246-250, 2000
6. Pogson DM, Doxey DL, Gilmour JS, Milne EM, Chisholm HK: Autonomic neurone degeneration in equine dysautonomia (grass sickness). J Comp Path 107:271-283, 1992
7. Summers BA, Cummings JF, De Lahunta A: Veterinary Neuropathology, pp. 433-434. Mosby, St. Louis, MO, 1995