Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 18
30 January, 2019
CASE I: 13-161654 (JPC 4048673)
Signalment: 25 year-old, female intact Congo African Grey Parrot (Psittacus erithacus erithacus)
History: This parrot was a permanent resident of a pet shop collection and was fed a diet of pellets, seeds, apples, and peanuts. The previous medical history included a prolapsed oviduct in May of 2013 with egg retention, but the bird was otherwise healthy. The bird was presented to the referring veterinarian in November of 2013 for a recent onset of lethargy, anorexia, and feather picking. On presentation, blood work abnormalities included increased bile acids and hyperproteinemia (absolute values were not provided).
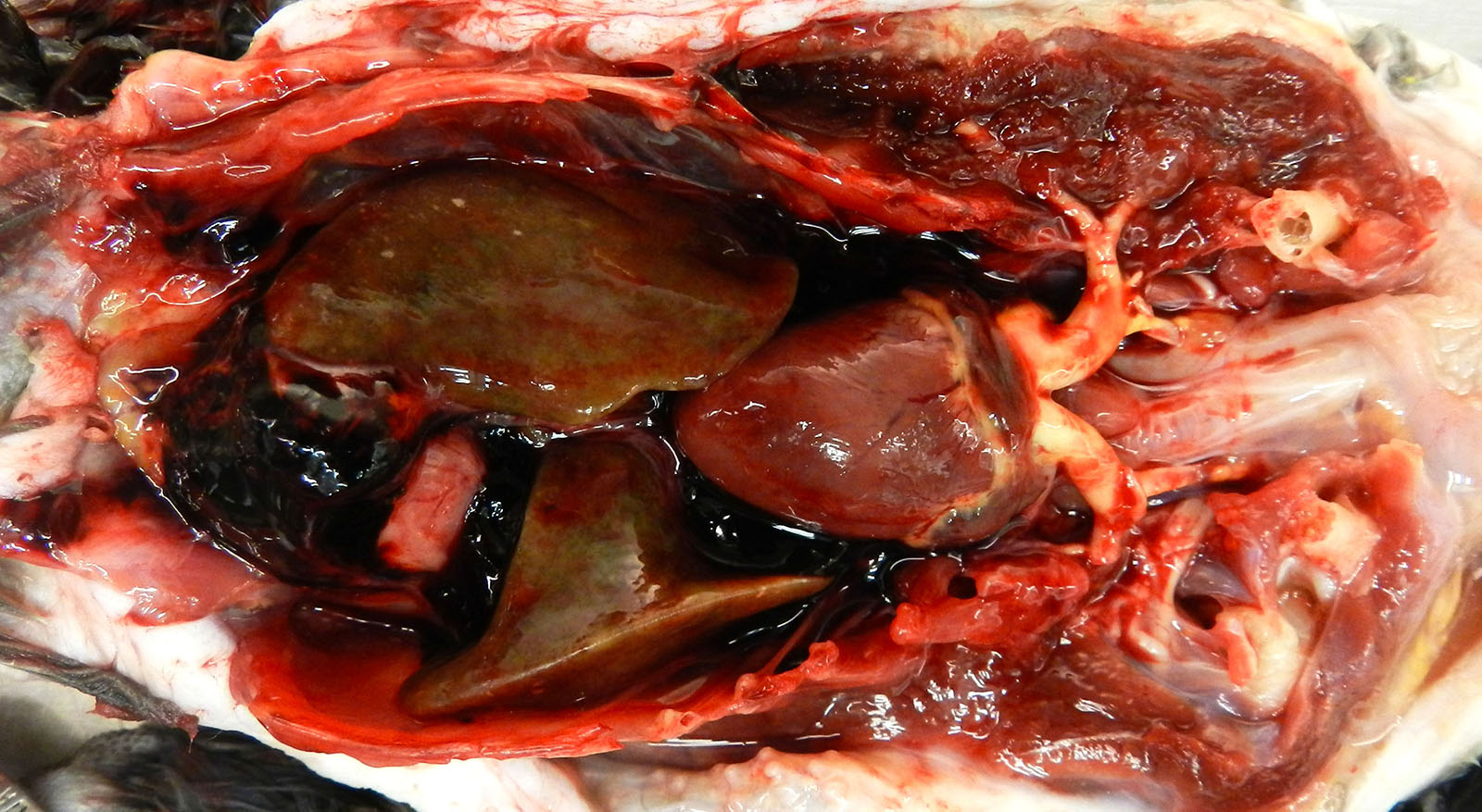
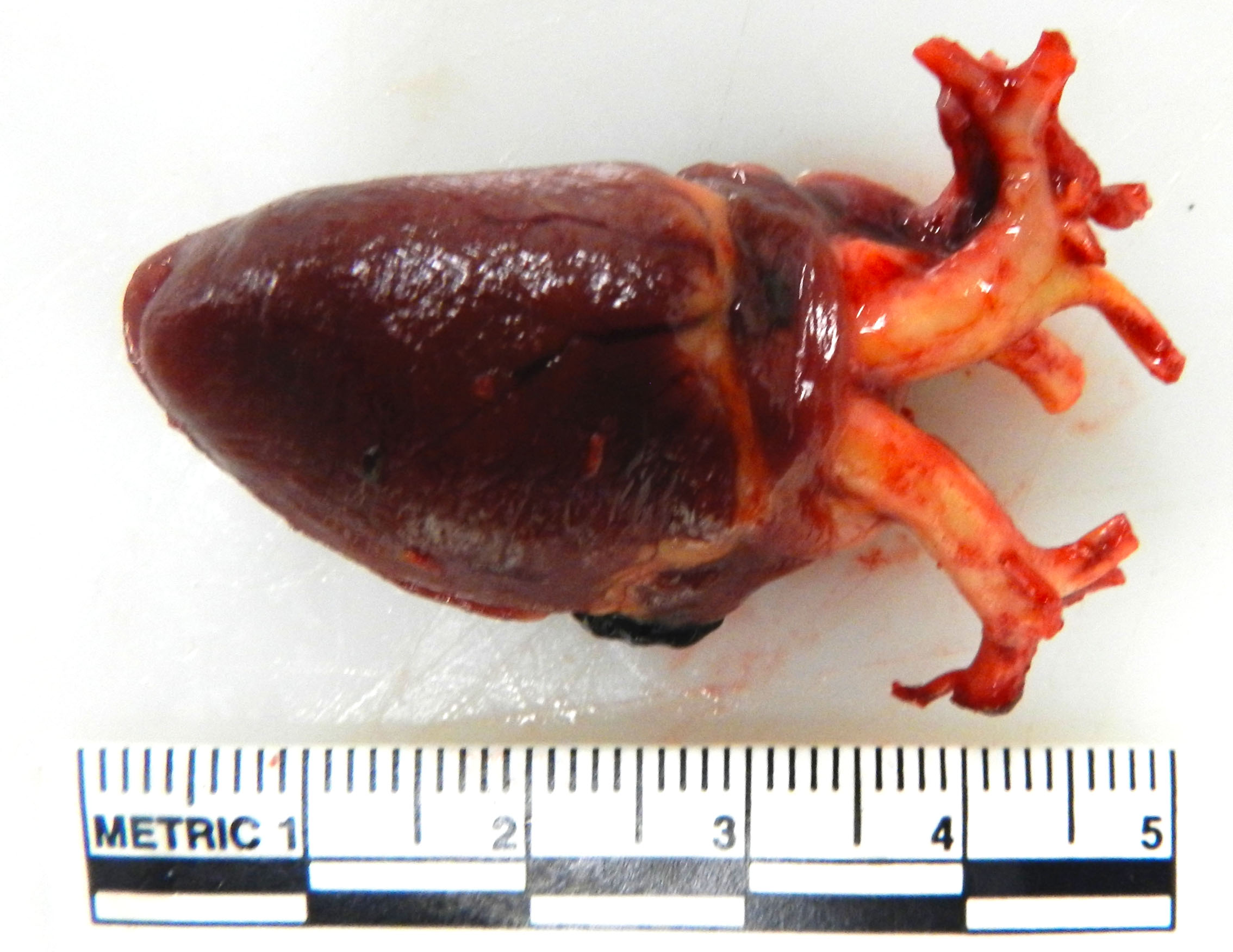
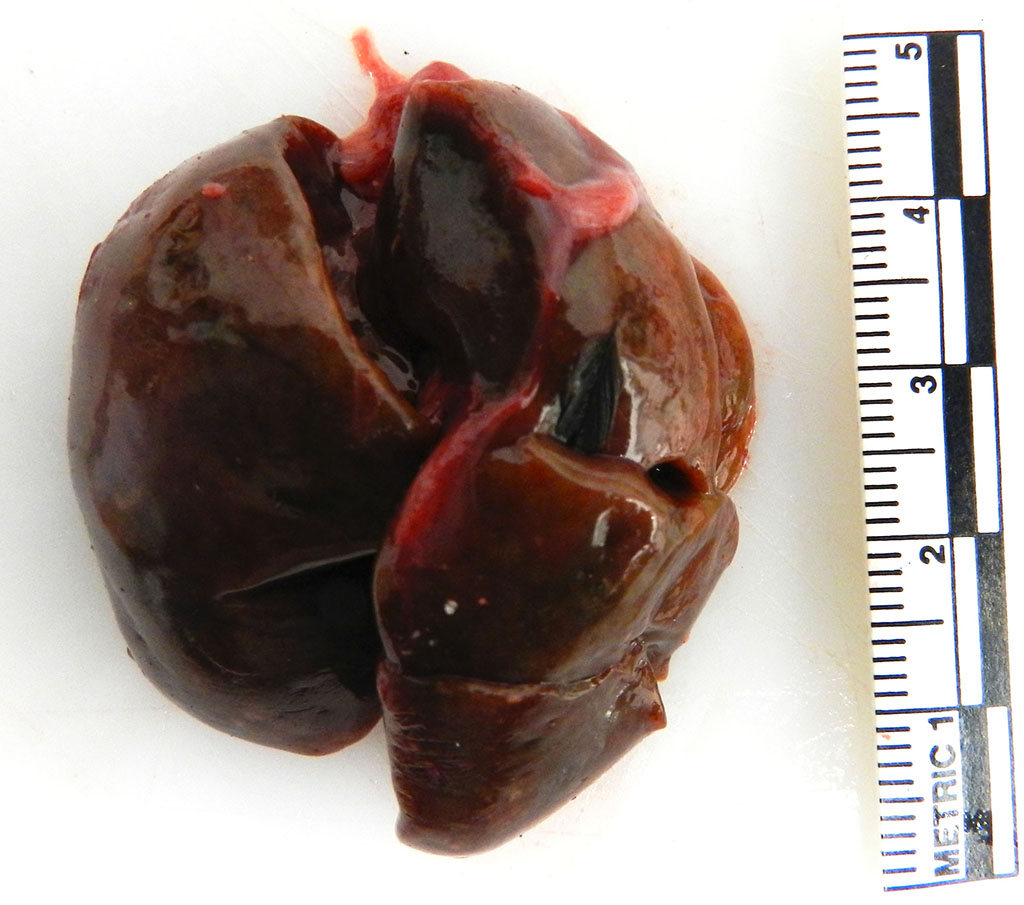
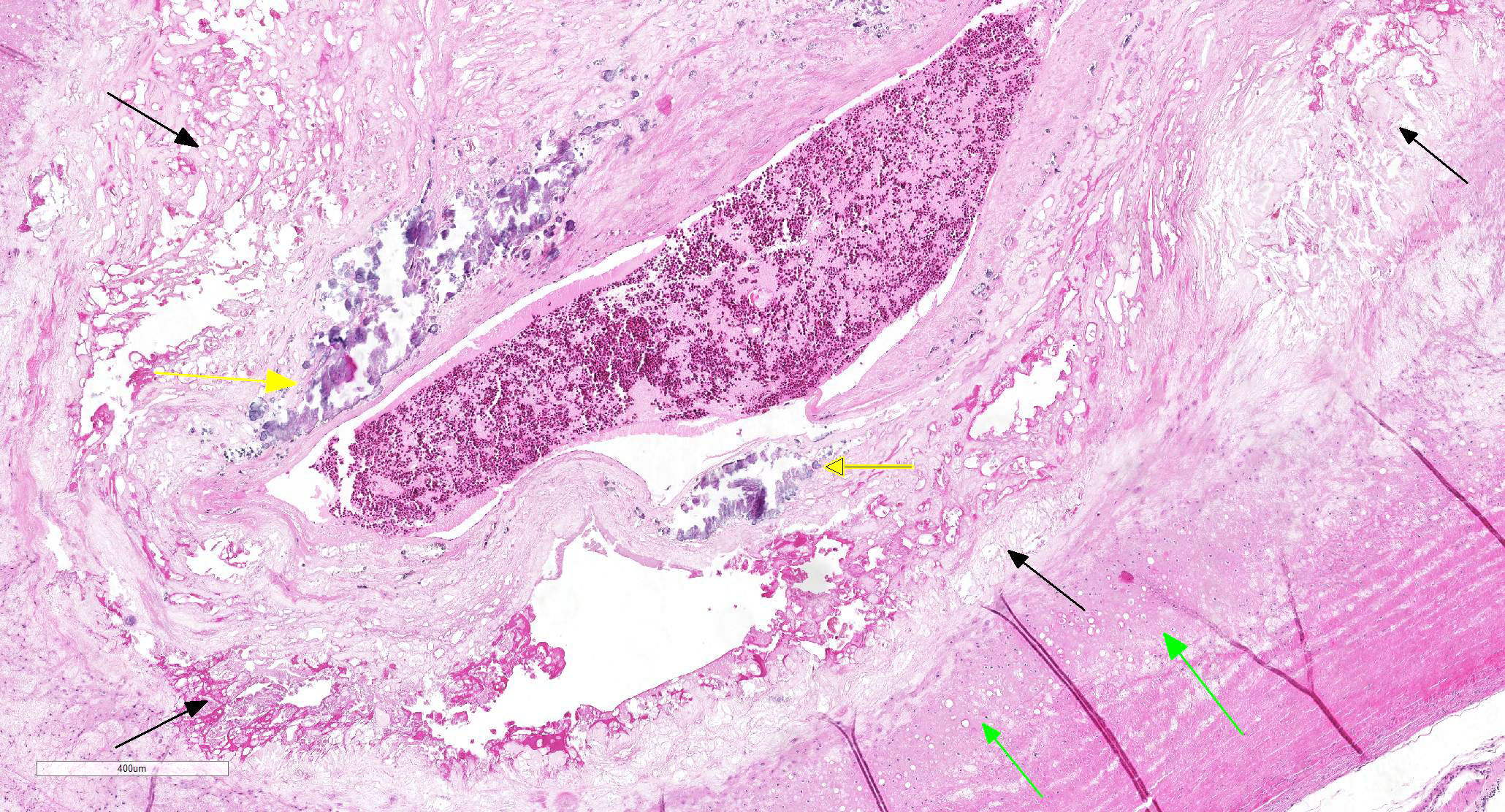
Gross Pathology: Both brachiocephalic arteries are prominent, diffusely yellow, hard and do not collapse. On section, the luminal surface is expanded by a locally extensive 1mm thick, rough, yellow plaque (atherosclerosis).
The coelomic cavity contains multiple aggregates of dark red gelatinous material that cover the viscera. The spleen is uniformly enlarged measuring 4.7 x4.5 cm and diffusely mottled white to light tan to red. The liver is enlarged with rounded margins and the parenchyma is diffusely mottled green to black to tan with dozens of random 0.1 cm round white foci (necrosis, presumptive).
Laboratory results: University of Miami – Avian and Wildlife Laboratory
- Increased beta and gamma globulins on electrophoresis consistent with acute inflammation and active humoral immune response
- High titer (1:25) for Chlamydophila psittaci
Microscopic Description:
HEART (not submitted): Randomly scattered throughout the parenchyma are small foci of cardiomyocyte loss with replacement by foamy macrophages and lymphocytes.
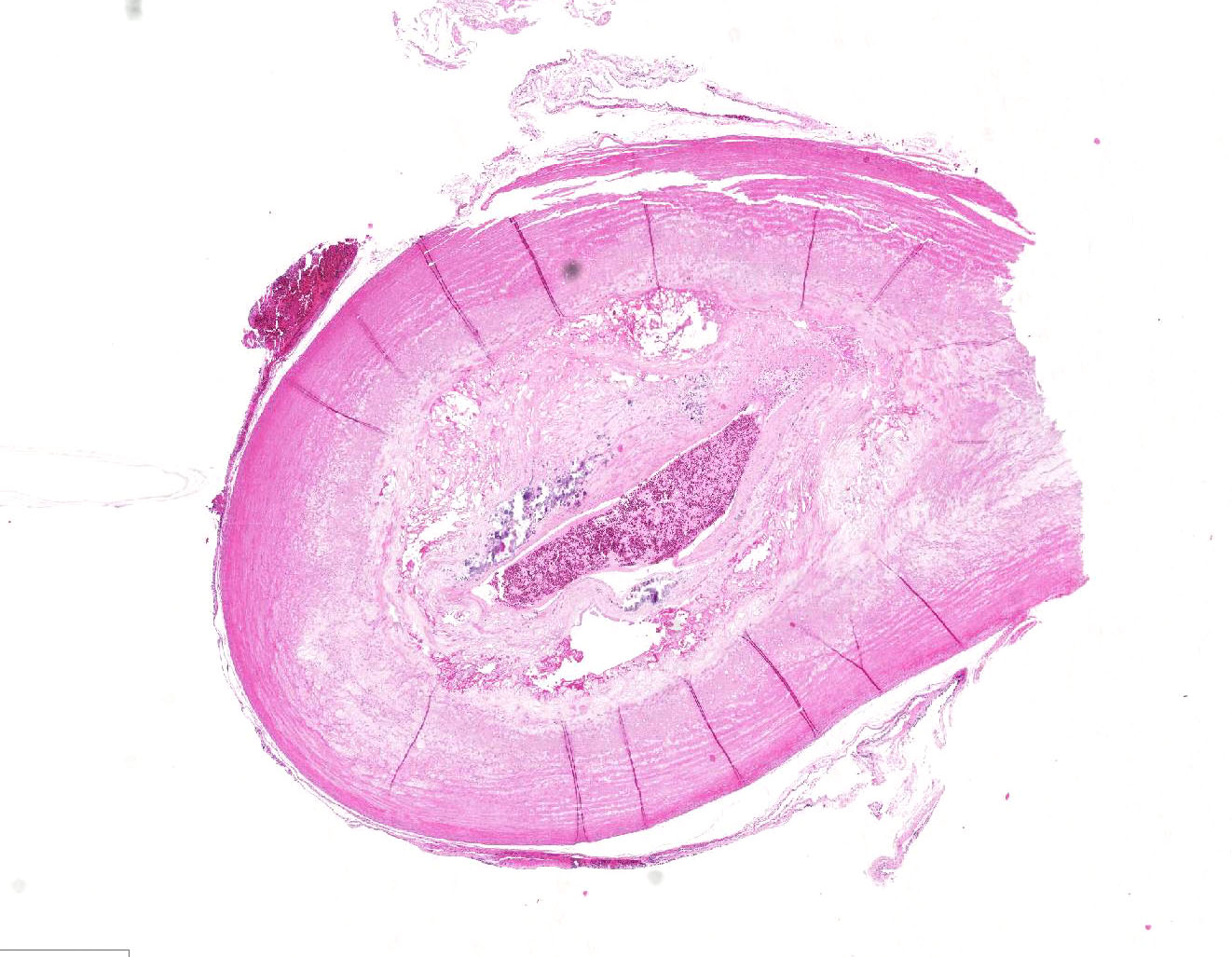
VESSEL, NOS: Arteries: Diffusely the tunica intima and media is disorganized with the tunica media expanded by abundant foam cells creating an irregular luminal surface. Occasionally the tunica media contains multifocal clear acicular clefts (cholesterol) that are variably surrounded by foamy macrophages and includes locally extensive regions of cellular loss with replacement by large clear spaces (fatty infiltrate) and several foci of chondroid metaplasia. The tunica intima and media contain numerous small aggregates of granular amorphous deeply basophilic material (mineral). In cross section the arteriolar lumen is occluded up to 60% by a confluent mass composed of a deeper region of large foam cells, acicular clear clefts (cholesterol), large clear spaces (fatty infiltration), multifocal aggregates of mineral, and islands of chondroid matrix covered by a thin variably present endothelial lining (atheroma).
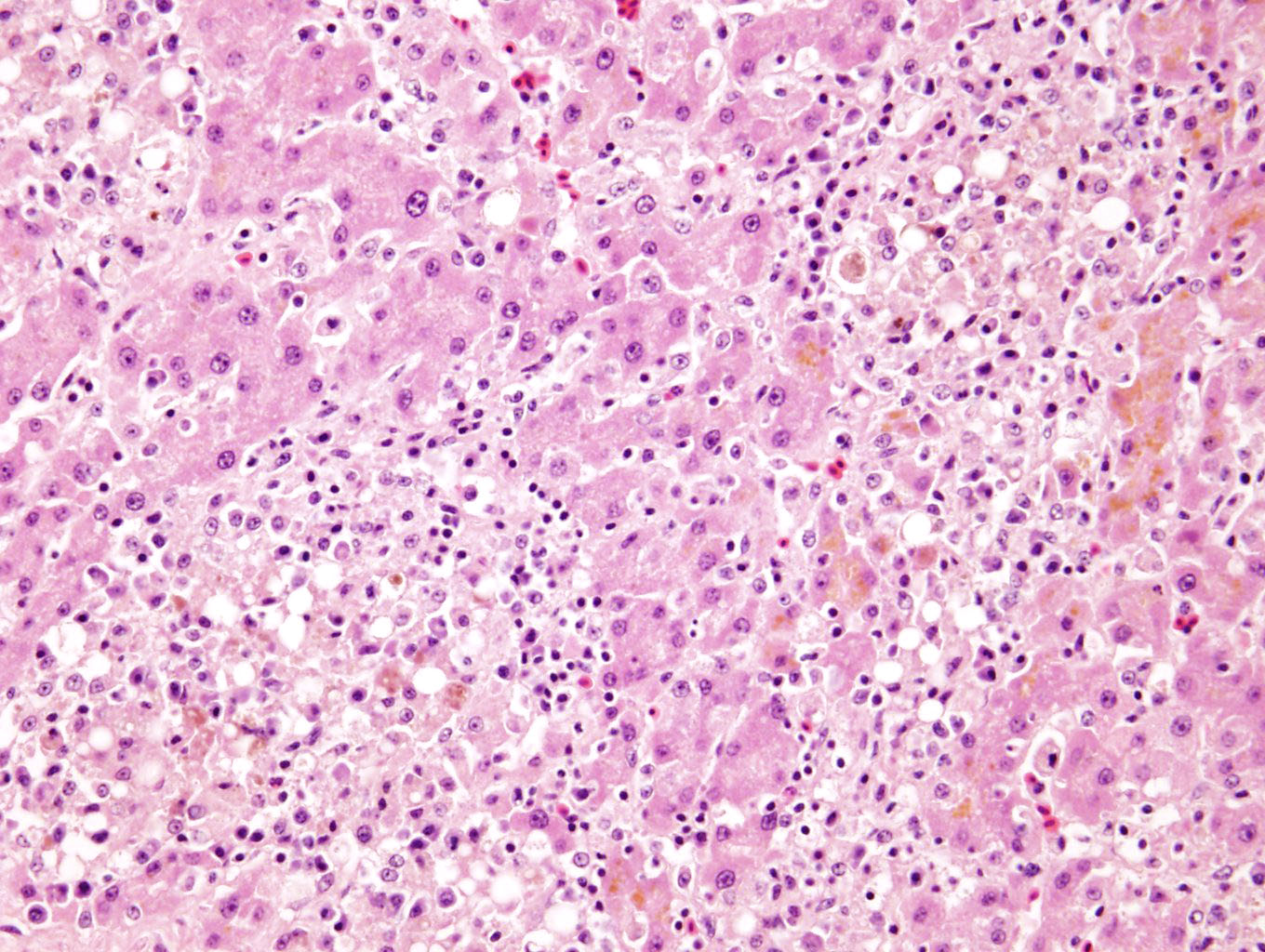
LIVER (not submitted): Randomly scattered throughout the hepatic parenchyma large coalescing areas of hepatocyte loss with the remaining hepatocytes being pale eosinophilic with loss of nuclear detail (necrosis). Admixed within these foci are small numbers of macrophages with fewer plasma cells and lymphocytes and rare heterophils. Surrounding hepatocytes contain fine granular golden brown cytoplasmic pigment (lipofuscin). The remaining hepatocytes show moderate anisocytosis and anisokaryosis, with occasional binucleate cells.
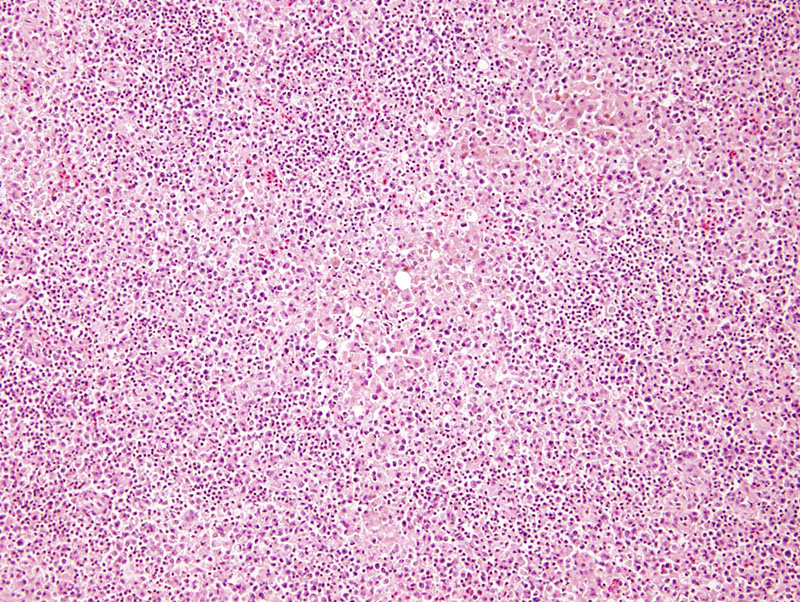
SPLEEN (not submitted): Diffusely expanding the red pulp are large coalescing aggregates of foamy macrophages admixed with few degenerate and non-degenerate heterophils.
Contributor’s Morphologic Diagnoses:
Arteries: Severe, diffuse, chronic atherosclerosis
Heart: Mild, multifocal, lymphohistocytic myocarditis
Liver: Severe, multifocal to coalescing, chronic, hepatocellular necrosis with granulomatous, lymphoplasmacytic hepatitis
Spleen: Severe, multifocal, chronic, granulomatous splenitis
Contributor’s Comment: The histologic features seen here are consistent with atherosclerosis of a grade IV out of VII, 5 although grade varies between submitted sections. The changes in the liver and spleen are seen with Chlamydophila psittaci infection, but other systemic bacterial infections (e.g. Escherichia coli, Salmonella sp, Mycobacterium sp, Staphylococcus sp, and Streptococcus sp) can create similar changes. Antemortem testing for Chlamydophila psittaci were consistent with an active infection. In this case the cause for coelomic hemorrhage remained unclear, as thrombotic events and vessel rupture are uncommon complications of atherosclerosis in the avian species.
Atherosclerosis is a chronic inflammatory fibroproliferative disorder, which commonly causes spontaneous disease in psittacines, as well as numerous other avian species. Most notably the Japanese Quail (Coturnix coturnix japonica) and pigeon (Columa livia domestica) have been used as inducible experimental models for the human disease.2,3,11 The pathogenesis of disease has been largely explained by the response-to-injury hypothesis. Here the principle inciting cause is endothelial damage, secondary to endothelial dysfunction and oxidative stress that creates a local atherogenic environment 2, 5. Endothelial dysfunction is characterized by a chronic state of endothelial cell activation leading to increased production of procoagulative and proinflammatory factors.2 In a recent study endothelial cell changes were appreciated in mild pre-atheroma lesions; giving further support to the hypothesis that endothelial damage precedes lipid deposition.5 A recent experimental model showed that type VIII collagen is up-regulated in artherosclerotic lesions and allows for smooth muscle cell proliferation and migration, which is important for fibrous cap formation.7
Currently the association between histologic severity and clinical disease course is unknown. As atherosclerosis in psittacines represents a similar spontaneous disease progression to that seen in humans, a similar grading scheme based on the American Heart Association’s human grading scheme has been purposed for better characterization and future prognostication of disease. This scheme divides atherosclerotic lesions into seven grades based on the light microscopic, histochemical, and electron microscopic features, with grades greater then IV of VII being considered clinically significant.5
Several risk factors have been proposed for the development of atherosclerosis in psittacines. Diet has been implicated, as many animal models for experimental disease are diet-inducible. In one study increasing the cholesterol within the feed of Quaker parrots (Myiopsitta monachus) by 1% induced clinically significant lesions within 4-6 months.6 With hypercholesterolemia, lipoproteins themselves can allow for activation of endothelial cells and leukocyte adhesion. Oxidized lipoproteins act as strong chemoattractants for monocytes. Once they enter the vascular wall and transform, to macrophages, they express scavenger receptors that allow for uptake of additional low density lipoproteins (LDLs) and simulation of smooth muscle cells to foam cells 2. In addition increased age, female sex, concurrent reproductive or hepatic disease, and the genera Psittacus, Amazona, and Nymphicus were found to increase the risk of development of severe atherosclerotic lesions.4 The synthesis and transport of VLDLs and vitellogenin to the oocyte and future egg yolk in response to estrogen may be the underlying pathogenesis for this predisposition in female birds.2 As VLDLs have been shown to be pro-inflammatory.2 Infectious agents have been implicated as risk factors for lesion development. In one study there was a significant association between Chlamydophilia psittaci antigen in the blood and development of atherosclerosis, however this correlation was not appreciated in one large retrospective study. However it is interesting to note that this patient had concurrent histologic findings and antemortem test findings consistent with active Chlamydophila psittaci infection. Marek’s disease (herpes virus infection) has been shown in chickens to promote atherosclerotic lesion formation.2
Underlying genetic factors can predispose a patient to endothelial dysfunction. In one study in White Carneau Pigeons there was a divergence in the genes responsible for cytoskeleton remodeling, proteasome activity, cellular respiration, and immune response. These genes were postulated to predisposition to the development of atherosclerotic lesions.1 The importance of gene divergence in other avian species has not been investigated.
Contributing Institution:
Cornell University – Animal Health and Diagnostic Center
240 Farrier Road
Ithaca, NY 14850
JPC Diagnosis:
- Elastic artery: Atherosclerosis, circumferential, diffuse, severe, with marked mural fibrosis, cartilaginous metaplasia and mineralization.
- Elastic artery, adventitial adipose tissue: Atrophy, diffuse, severe.
JPC Comment: The contributor has provided an excellent discussion of atherosclerosis in psittacines and other avian species, highlighting a number of abnormalities which are involved in this complex condition. Species such as humans, non-human primates, pigs, rabbits and hamsters are considered susceptible to atherosclerosis and its predisposing factor, hypercholesterolemia. Other laboratory animals such as mice, rats, dogs and shrews exhibit resistance to these conditions.9
The recent literature abounds with studies on various areas of research into animal models of atherosclerosis, in a number of areas of current interest in the pathogenesis of human atherosclerosis – genetic abnormalities, the contribution of inflammation and inflammatory mediators on the development of atheromatous plaques, endothelial dysfunction, and diet/dyslipidemia. Genetic abnormalities, such as the deficiency of low-density lipoprotein receptors (LDLR), are widespread in humans (with over 1700 separate genetic mutations to date)9 and have been a subject of investigation since the use of the Watanabe rabbit in the 1970’s. Today, genetic manipulation of knockout mice and rabbits involved in the pathway of production and degradation of the LDLR continues to further research in this area.9
Diet-induced atherosclerosis (or enhancement of disease in atherosclerosis –resistant species) is a time-honored research avenue which is still being applied today to new animal models. The Gottingen minipig has been a model for atherosclerosis for the last decade. Diets high in fat/cholesterol and cholate have the potential for inducing advanced coronary and atherosclerotic lesions in the species10, as has been demonstrated in a number of other species over the years, to include laboratory rodents as well as non-human primates.
An excellent review from a species perspective on atherosclerosis was part of a recent review of aging changes in great apes.8 In contrast to early literature stating that chimpanzees were suitable animal models for coronary atherosclerosis (especially if subjected to atherogenic diets), the actual incidence of coronary atherosclerosis is quite low in apes in well-managed colonies and collection; however more data needs to be collected on aortic atherosclerosis in these species.8
We are unable to confirm the diagnosis of chlamydia in this case, as liver and splenic tissue were not part of the submission. The connection between chronic inflammation and atherosclerosis is well-documented in the literature; however, the connection between the two lesions in this animal and its significance would be very difficult to assess.
Early on in the discussion, the moderator pointed out the difficulty in even establishing that whether this section represented an elastic or a muscular artery. Without the use of special stains, the mural changes make it difficult to visually quantitate the amount of elastin within the wall. Muscular arteries, which deliver blood to specific organs, tend to have walls composed primarily of smooth muscle, while elastic arteries have a higher concentration of elastic fibers. Both the moderators from this week and last week conference agreed that the differentiation of elastic versus muscular arteries is extremely difficult in very diseased vessels.
The moderator reviewed atherosensitive species including rabbits, guinea pigs, birds, and pigs, as well as atheroresistant species - dogs, cats, cattle, goats, and unmanipulated mice/rats. In psittacines, the disease disease is common in Amazons, African greys, cockatiels and Quaker parrotss. The participants also discussed the response to injury (endothelial damage and the generation f a procoagulative and proinflammatory state) versus the response to retention theory (binding of LDL’s by arterial extracellular matrix and promotion of further endothelial damage and recruitment of additional lipids and leukocytes) in atherogenesis. The moderator also reviewed the currently published grading system for atherosclerosis in psittacines.6
References:
- Anderson JL, Ashwell CM, Smith SC, et al. Atherosclerosis-susceptible and atherosclerosis-resistant pigeon aortic cells express different genes in vivo. Poultry Science. 2013;92(10):2668-80. doi: 10.3382/ps.2013-03306.
- Beaufrère H. Atherosclerosis: Comparative Pathogenesis, Lipoprotein Metabolism, and Avian and Exotic Companion Mammal Models. Journal of Exotic Pet Medicine. 2013;22(4):320-35. doi: 10.1053/j.jepm.2013.10.016.
- Beaufrère H. Avian Atherosclerosis: Parrots and Beyond. Journal of Exotic Pet Medicine. 2013;22(4):336-47. doi: 10.1053/j.jepm.2013.10.015.
- Beaufrère H, Ammersbach M, Reavill DR, et al. Prevalence of and risk factors associated with atherosclerosis in psittacine birds. Journal of the American Veterinary Medical Association. 2013;242(12):1696-704. doi: 10.2460/javma.242.12.1696.
- Beaufrere H, Nevarez JG, Holder K, et al. Characterization and classification of psittacine atherosclerotic lesions by histopathology, digital image analysis, transmission and scanning electron microscopy. Avian pathology : Journal of the WVPA. 2011;40(5):531-44. doi: 10.1080/03079457.2011.607427. PubMed PMID: 21879992.
- Beaufrere H, Nevarez JG, Wakamatsu N, et al. Experimental diet-induced atherosclerosis in Quaker parrots (Myiopsitta monachus). Veterinary pathology. 2013;50(6):1116-26. doi: 10.1177/0300985813488958. PubMed PMID: 23696447.
- Lopes J, Adiguzel E, Gu S, et al. Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am J Pathol. 2013;182(6):2241-53. doi: 10.1016/j.ajpath.2013.02.011. PubMed PMID: 23567639; PubMed Central PMCID: PMC3668035.
- Lowenstine LJ, McManamon R, Terio KA. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet Pathol 2016: 53(2): 250-256.
- Lu R, Yuan T, Wang Y, Zhang T, Yuan U, Wu D, Zhow M, He Z, lu Y, Chen YU, Fan J, Liang J, Cheng Y. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabits with deficiency of low-density lipoprotein receptor (LDLR0 on exon 7. Ebiomedicine 36(2018) 29-38.
- Ludvigsen TP, Kirk KE, Christofferssen BO, Pedersen HD, Martinussen T, Kildegard JU, Heegaard PMH, Lykkesfeldt J, Olsen LH. Gotten miniid model of diet-induced atherosclerosis: influence of mild streptozotocin-induced diabretes on lesion severity and markers of inflmmation evaluated in obese, obese and diabetic, and lean control animals. J Translat Med' 2015; 13:312-324.
- Pilny AA, Quesenberry KE, Bartick-Sedrish TE, Latimer KS, Berghaus RD. Evaluation of Chlamydophila psittaci infection and other risk factors for atherosclerosis in pet psittacine birds. JAVMA. 2012;240(12):1474-80.