Signalment:
Gross Description:
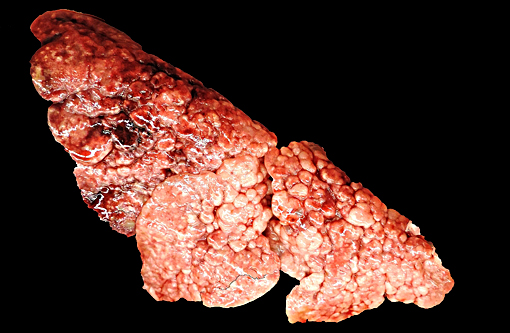
All lung lobes were firm and displayed a prominent nodular pattern, with nodules ranging from 0.2 cm to 0.5 cm in diameter. The pancreas consisted of discrete, multifocal, 3 mm diameter and smaller, white to tan nodules. The splenic capsule contained few, multifocal, 2 mm diameter white to tan, firm, slightly raised patches. The right thyroid gland was moderately enlarged relative to the left.
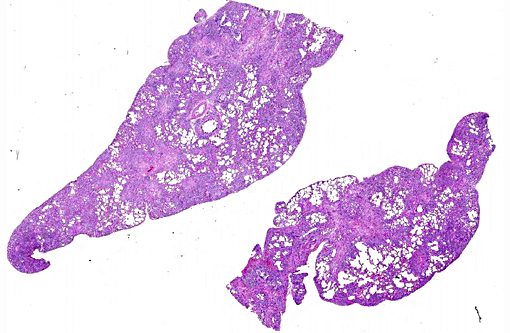
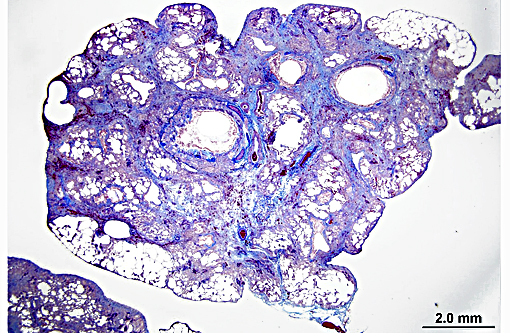
Histopathologic Description:
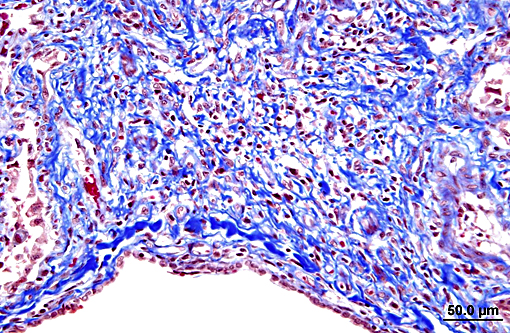
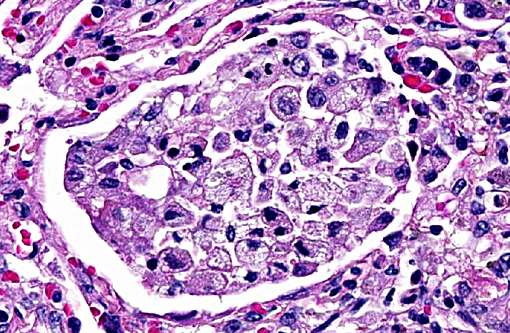
Terminal bronchiolar smooth muscles are frequently markedly hyperplastic and remaining alveoli often filled with foamy macrophages and fewer lymphocytes, plasma cells and rare neutrophils. Alveoli are also often lined by plump cuboidal pneumocytes (type II pneumocyte hyperplasia).
Within bronchiolar and bronchial lumens, is abundant basophilic, slightly fibrillar material (mucus) admixed basophilic nuclei and nuclear debris (necrotic neutrophils). Bronchial glands are mildly hyperplastic. The tunica media of vessels is markedly thickened (hyperplastic).
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Diagnosis of human IPF relies on the exclusion of other known causes of interstitial lung disease (ILD), the presence of a UIP pattern on high resolution CT scans (HRCT), or specific combinations of HRCT and lung biopsy patterns in patients where lung biopsy samples are obtained.(4)
Human IPF carries a variable and unpredictable natural history, and remains a poorly understood. While an exact etiology for human IPF remains unknown, several risk factors have been identified, including cigarette smoking, environmental exposures (metal and wood dusts, farming, raising birds, hairdressing, stone polishing), microbial agents (Epstein-Barr virus, hepatitis C virus), and gastroesophageal reflux.(4) Genetic factors have also been identified in the context of human IPF. For example, strong associations with familial IPF have been identified with mutations in the surfactant protein C gene.(6)
Animal models used to study IPF often fail to mimic morphological changes seen in human IPF, nor do they follow a similar progression of disease.(7) Beginning in 1996, IPF has been recognized in cats,(1) characterized as a novel spontaneous chronic, progressive respiratory disease with morphologic features of UIP.(2) One study found that the average age of onset is 8.7 years, and average duration between onset of disease and death/euthanasia is 5.5 months.(7) The disease in cats shares critical features with human IPF in the context of gross pathology, histopathology, cell differentiation markers, and ultrastructural details.(5,7) No other spontaneous disease of induced animal model is able to reproduce the criteria for UIP as in feline IPF.(2)
Grossly, lungs are mottled tan to red, and display a distinct cobblestone appearance on pleural surfaces that typically involves large regions of the lungs. Additionally, the extensive areas of fibrosis typically form plaque-like regions that are discernable from normal lung tissue and extend deep into the parenchyma. Grossly discernable honeycombing is an uncommon finding.(7)
The histopathologic hallmark (and most important diagnostic criterion) is that of a heterogeneous appearance at low magnification, characterized by fibrosis with scarring and honeycomb change that alternate with regions of less affected or even normal parenchyma.(3,4,5,7) Other changes include foci of fibroblasts/myofibroblasts, and interstitial smooth muscle hyperplasia.(1, 2) The amount of inflammation is variable, but not a prominent feature, however, large foci of alveolar macrophages is a common finding. In some (69%) cats, mucous cell metaplasia is noted. In cats that do not demonstrate mucous cell metaplasia, the lining cells are either type II pneumocytes (well differentiated) or columnar cells of an unknown phenotype.(7)
In typical cases, lesions are often patchy or multinodular, rather than diffuse. The histopathologic changes often preferentially affect the subpleural and paraseptal parenchyma.(3,4) This case is atypical in that lesions efface the entire pulmonary parenchyma, with virtually no normal tissue remaining. Similar cases have been documented by other authors, with the conclusion that the disease was too advanced to appreciate any temporal heterogeneity.(3) Similarly, this case likely represents advanced disease, as this animal survived approximately one year after detection of clinical signs (average duration is 5.5 months). This animal died after a traumatic encounter with a dog. Thus, the cause of death was concluded to be respiratory compromise in the face of increased respiratory demand due to the traumatic incident.
JPC Diagnosis:
Lung: Fibrosis, interstitial, multifocal to coalescing, marked with smooth muscle hypertrophy and hyperplasia, type II pneumocyte hyperplasia, and moderate alveolar histiocytosis.
Lung: Bronchitis and bronchiolitis, chronic-active, multifocal, moderate with squamous metaplasia.
Conference Comment:
Most agreed that the mostly neutrophilic inflammatory infiltrate within the larger airways along with mucous exudation was a secondary process and may have been related to the positive bacterial culture in the lungs.
The precise pathogenesis of feline idiopathic pulmonary fibrosis is unclear, but current scientific literature suggests a defect in type II pneumocytes and alveolar repair are precursory findings. The loss of these cells may have an important role in the development of pulmonary fibrosis.(7) Chronic interstitial inflammatory stimulation resulting in fibrosis has also been implicated in cases of interstitial fibrosis. However, in feline idiopathic pulmonary fibrosis, inflammation may not have an essential role and the primary defect and stimulus may involve the type II pneumocytes. In this case, conference participants noted that, overall, interstitial inflammation was mild at best and not a significant feature. Additionally, ultrastructural changes suggest surfactant protein C may play a role in the disease.(7)
This case was submitted to the Department of Pulmonary and Mediastinal Pathology at the Joint Pathology Center (JPC) for comparative pathology consultation regarding idiopathic pulmonary fibrosis in humans. Their consultation revealed similar histologic features as those discussed in the conference, but proposed an alternative interpretation to these changes. Their interpretation is that of an organizing pneumonia characterized by airway luminal, airspace, and interstitial loose fibroblastic connective tissue. Though difficult to discern due to overwhelming and diffuse injury, they favored an airway-centered process and described organizing pneumonia, in general terms, as a subacute process that is most commonly the sequelae of infection and, in many cases, difficult/impossible to document. The larger cartilaginous airways were described as showing submucosal and adventitial chronic inflammation with varying degrees of destruction including complete obliteration of the small airways. In their opinion, this case resembles progression of subacute organizing pneumonia to non-specific interstitial pneumonia (with fibrosis) with the histomorphologic features less consistent with unusual interstitial pneumonia (UIP) as it occurs in people. However, also noted in their detailed consultation, interstitial pulmo-nary fibrosis in cats (i.e. a novel spontaneous chronic, progressive respiratory disease with morphologic features of UIP in humans) may have variations in histologic appearance as compared to humans suggesting possible species differences.
References:
1. Caswell JL, Williams KJ. Respiratory system. In: Maxie, MG ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:570.
2. Cohen LA et al. Identification and Characterization of an Idiopathic Pulmonary Fibrosis-Like Condition in Cats. J Vet Intern Med. 2004; 18: 632-641.
3. LeBodec K, Roady P, OBrien RT. A Case of Atypical Diffuse Feline Fibrotic Lung Disease. Journal of Feline Medicine and Surgery. 2014; 16(10): 858-863.
4. Raghu et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence Based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011; 183: 788-824.
5. Rhind SM and Gunn-Morre, DA. Desquamative Form of Cryptogenic Fibrosing Alveolitis in a Cat. J Comp Path. 2000; 123: 226-229.
6. Thomas AQ, et al. Heterozygosity for a Surfactant Protein C Gene Mutation Associated with Usual Interstitial Pneumonitis and Cellular Nonspecific Interstitial Pneumonia in One Kindred. Am J Respir Crit Care Med. 2002; 165: 1322-1328.
7. Williams K et al. Identification of Spontaneous Feline Idiopathic Pulmonary Fibrosis: Morphology and Ultrastructural Evidence for a Type II Pneumocyte Defect. Chest. 2004; 125: 2278-2288.