Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 20
25 March, 2020
CASE I: T-1708131 (JPC 4117493).
Signalment: Seven-month-old, female, domestic short hair cat, Felis catus
History: The animal presented to the referring veterinarian for general malaise and chronic weight loss. The cat returned home with the owner and was subsequently found dead a few days later. The carcass was submitted to the Tifton Veterinary Diagnostic and Investigational Laboratory (TVDIL) for necropsy.
Gross Pathology: At necropsy, the musculature was mildly atrophied. The oral mucosa was pale beige to grey. Lung lobes were mottled dark red to beige with frequent pinpoint pale foci. Liver had numerous 1 mm to 25 mm in diameter, slightly firm, pale foci. Multifocal to coalescing, irregular, and yellow beige foci, ranging from 2 to 20 mm in diameter were also observed on renal capsule and in renal cortices. Mesenteric lymph nodes were markedly enlarged. There was no other grossly visible lesion at necropsy.
Laboratory results: Bacterial culture on the lung and liver yielded no bacterial growth. Fluorescent antibody tests on lung, spleen, and kidney were negative for both feline infectious peritonitis and feline leukemia virus. PCR for feline coronavirus on pooled samples from lung, kidney, liver, and brain was positive.
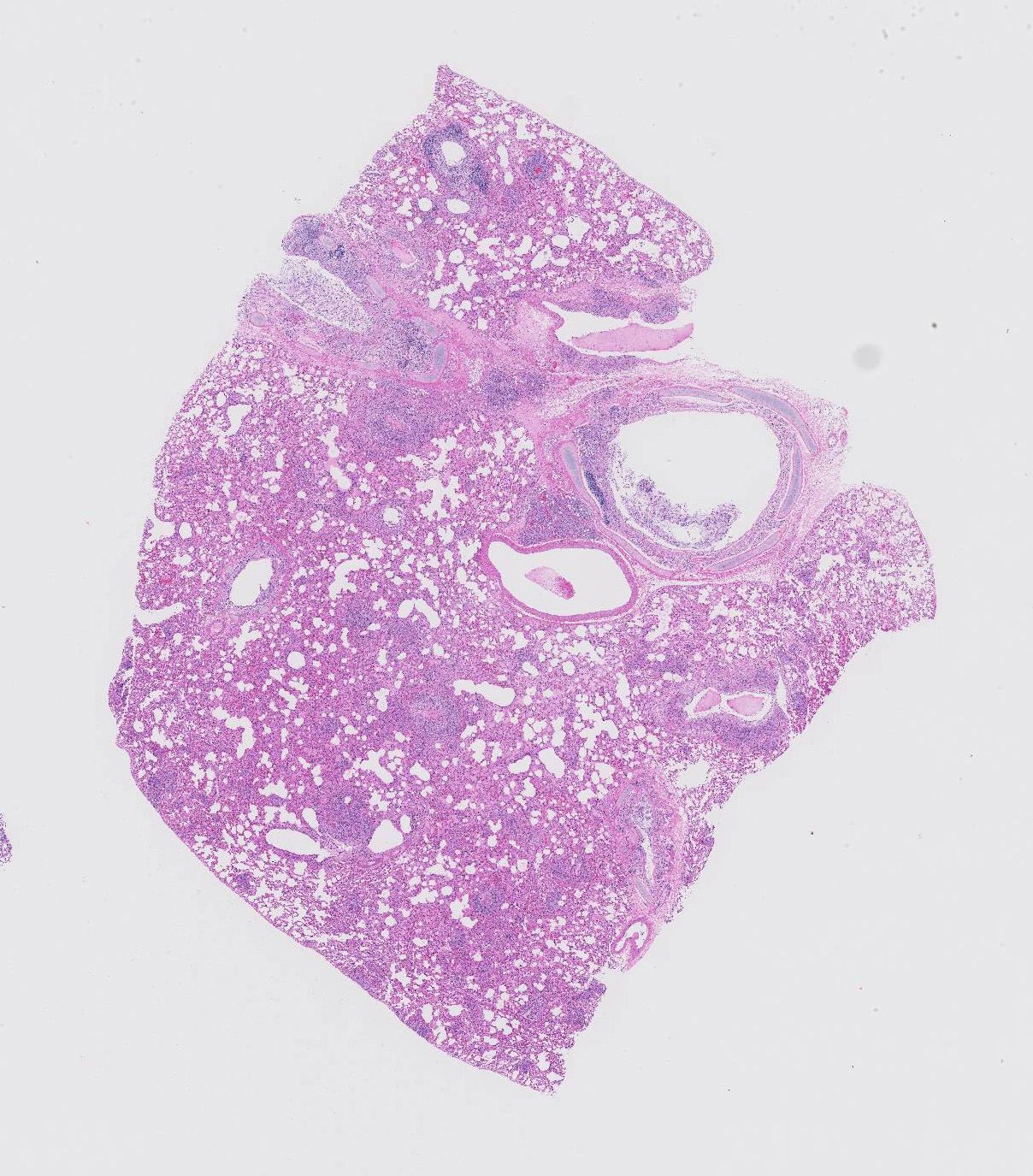
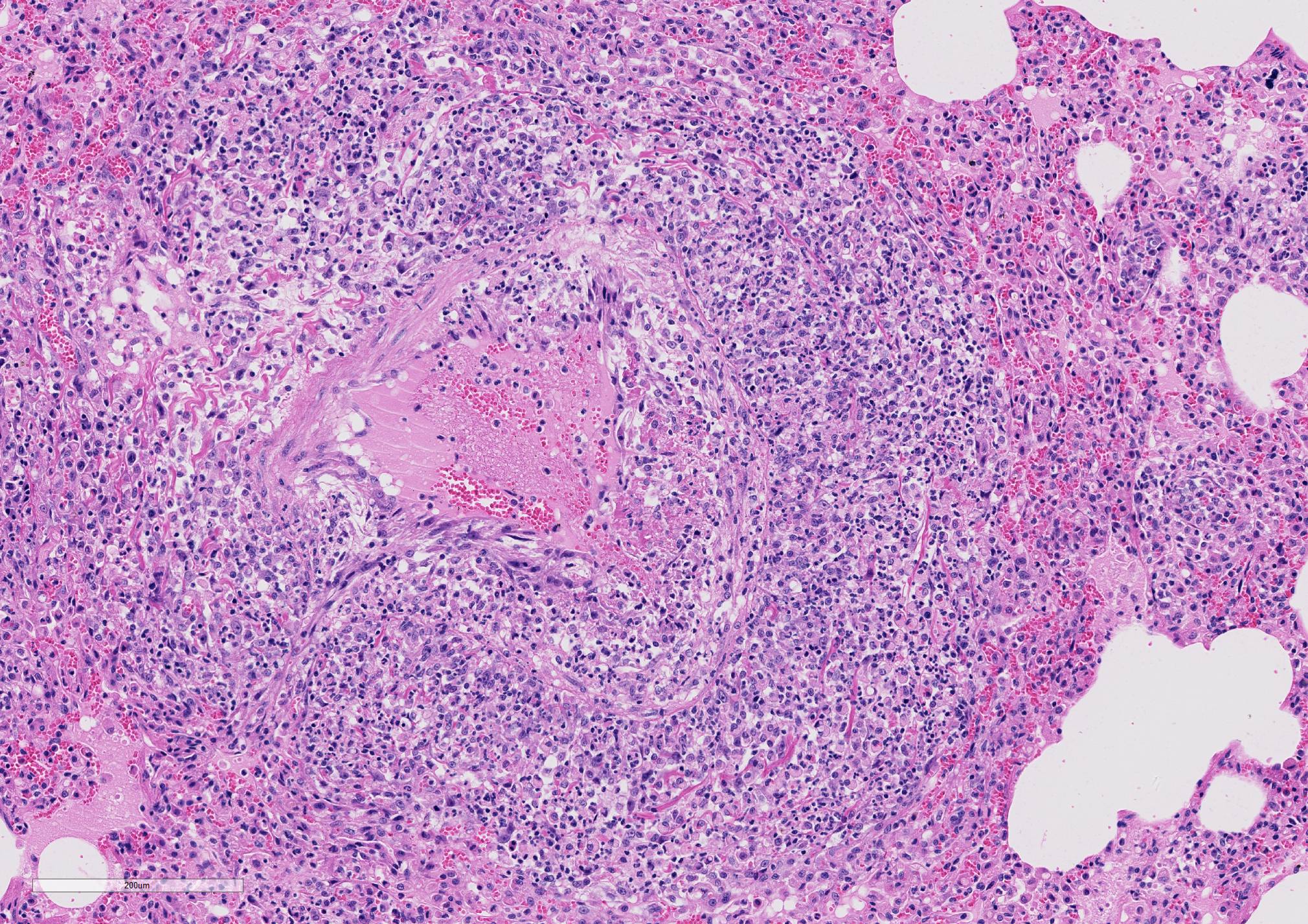
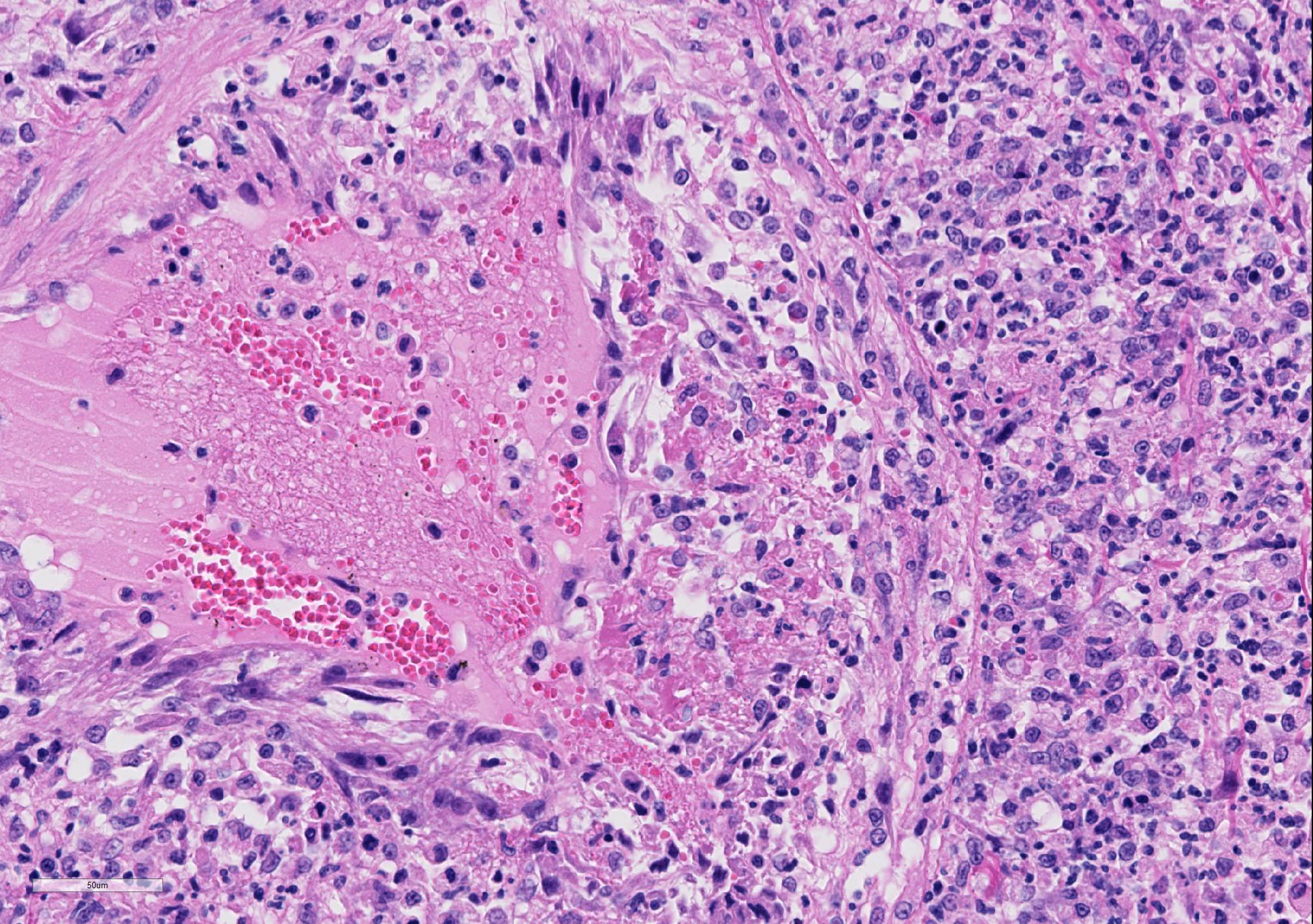
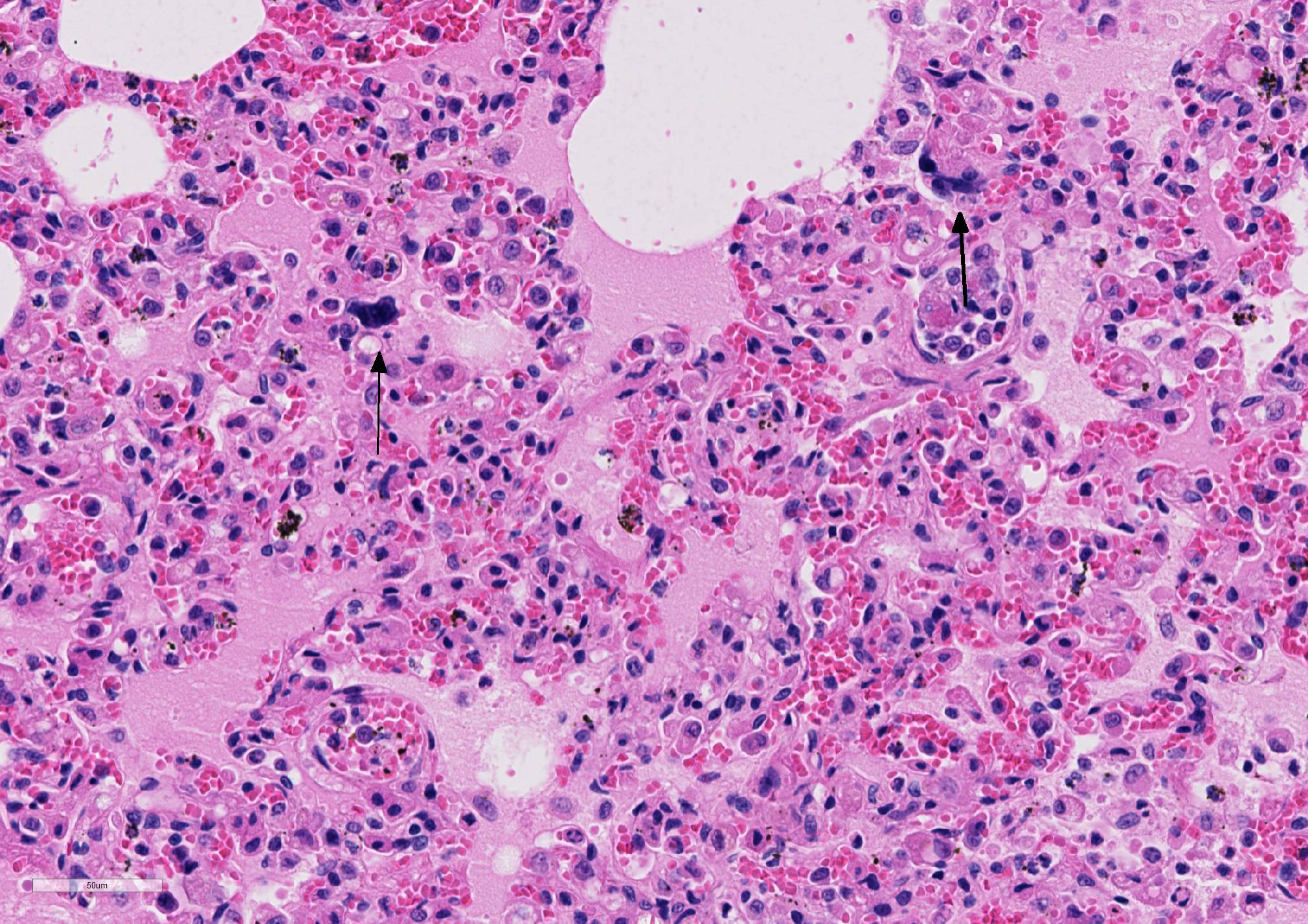
Microscopic Description: Lung sections were congested and disrupted by multifocal to coalescing areas of marked inflammation composed of large numbers of macrophages and neutrophils admixed with fewer plasma cells and lymphocytes. Occasional inflammatory foci contained multinucleated giant cells. Multifocally, tunica media and tunica intima of the pulmonary vessels were disrupted and expanded by an eosinophilic fibrillar material, degenerate neutrophils, and karyorrhectic debris. Scattered vessels were partially occluded by thrombi. Alveoli often contained an eosinophilic material admixed with low to moderate numbers of macrophages. A few bronchioles and bronchi contained an eosinophilic wispy material admixed with low numbers of macrophages and neutrophils. The peribronchiolar lymphoid tissue was hyperplastic. The pleural mesothelium exhibited mild hypertrophy. In a few regions, the pleural surface was overlaid with fibrin mats that contained degenerate neutrophils (not present in all sections).
Contributor Morphologic Diagnosis:
1. Pneumonia, interstitial, pyogranulomatous, subacute to chronic, multifocal to coalescing, marked with vasculitis, lung.
2. Nephritis, interstitial, pyogranulomatous to plasmacytic, subacute to chronic, multifocal to coalescing, marked with vasculitis, kidney (not present in slide).
3. Hepatitis, pyogranulomatous to plasmacytic, subacute to chronic, multifocal to coalescing, marked, liver (not present in slide).
4. Meningoencephalitis, neutrophilic and histiocytic to plasmacytic, subacute to chronic, multifocal, marked with vasculitis, brain (not present in slide).
5. Lymphadenitis, pyogranulomatous to plasmacytic, subacute to chronic, multifocal to coalescing, marked, mesenteric and splenic lymph nodes (not present in slide).
6. Myocarditis, necrotizing to pyogranulomatous, subacute to chronic, multifocal, moderate to marked, heart (not present in slide).
7. Cystitis, interstitial, pyogranulomatous, subacute to chronic, multifocal, moderate to marked, urinary bladder (not present in slide).
Contributor Comment: Histopathology confirmed a marked pyogranulomatous interstitial pneumonia with vasculitis. Similar foci of pyogranulomatous inflammation and vasculitis were observed in the kidney, liver, brain, lymph nodes, heart, and urinary bladder. The pattern of multisystemic pyogranulomatous inflammation with vasculitis was consistent with the noneffusive form of feline infectious peritonitis (FIP). PCR on fresh samples of lung, liver, kidney, and brain were positive for feline coronavirus. These results in conjunction with the histopathologic lesions confirmed FIP.
FIP is a fairly common infectious disease of cats with a low morbidity, yet high mortality. FIP is a coronaviral disease that can affect cats of any age, but is most prevalent among cats <3 years of age and especially from 4 to 16 months of age.4 The disease continues to be a major killer of young cats.3 In general, FIP tends to affect young cats, but can still occur in middle-aged to older cats. Purebred cats and male cats appear to be more susceptible.4 It has also been well documented in virtually every species of Felidae and FIP of domestic cats and cheetahs has been historically intertwined.2,4
Coronaviruses have adapted themselves over thousands of years to virtually every species of mammals and birds, and are a common cause of transient enteritis and respiratory disease.3 The causative agent of FIP is feline coronavirus and more specifically, the feline infectious peritonitis virus (FIPV) biological pathotype. The virus is an Alphacoronavirus in the family Coronaviridae.5 The other pathotype being feline enteric coronavirus (FECV), which is restricted to the intestinal tract and generally considered to be avirulent.5 The exact pathogenesis of the viral infection and development of clinical disease is not fully elaborated, but the ability of the virus to replicate within macrophages is important in the development of FIP.5,6 Mutation of FECV to a virulent FIPV, has been considered as a possible contributing factor to the development of feline infectious peritonitis.4,5,6 Other factors contributing to FIP include viral strain, genetic predisposition, and the host?s immune response (e.g. ineffective cell mediated immunity of the host will favor an FIP virus infection).5,6 The role of genetic factors in resistance or susceptibility to FIP is based on both indirect and direct observations. Pedigreed cats are more likely to develop FIP than random-bred cats and certain breeds are also more severely affected than others.3
Resistance to FIP is complicated and involves genetic susceptibility, age at the time of exposure and a number of stressors that occur at the same time as infection and have a negative impact on the ability of the infected cat to eliminate the virus. The time period between initial FECV exposure and clinical signs of disease can be as short as 2?3 weeks, as long as several months or, rarely, years, reflecting the time it takes for mutant FIPVs to evolve, or for the infection to progress from a subclinical to clinical disease. After an onset of overt clinical disease a return to normal health is extremely uncommon and rarely may a cat will make an apparent recovery, but only to have clinical signs recur months and even years later. The disease course is generally shorter in younger cats and cats with effusive disease than in older cats and cats with non-effusive disease.3
FIP manifests itself in two forms the noneffusive (?dry?) form as displayed in this case and the effusive (?wet?) form. Despite the distinct nomenclature, these forms likely represent the two extremes of a spectrum. The effusive form typically presents with multiple acutely developing effusions in body cavities predominantly the abdominal and pleural cavities. Also serosal surfaces often have fibrin deposits with necrotic foci in the associated parenchyma.5 The noneffusive form however is chronic and characterized by multisystemic vasculitis and perivascular inflammation. The inflammatory infiltrate is classically histiocytic to pyogranulomatous with variable numbers of neutrophils, plasma cells and lymphocytes.5The lesions are commonly observed in the kidney, eye, brain, lung, liver, lymph nodes, and serosal surfaces. Grossly, the noneffusive form off FIP can present similar to systemic bacterial, fungal or protozoal infections and disseminated neoplasia, especially lymphoma.5
Diagnosis of FIP is based first and foremost on consideration of the age of the patient, origin, clinical signs and physical examination. Abdominal distension with ascites, dyspnea with pleural effusion, jaundice, hyperbilirubinuria, discernible masses on the kidneys and/or mesenteric lymph nodes, uveitis and a range of neurological signs associated with brain and/or spinal cord involvement are all common in cats with either the effusive or non-effusive form of FIP. At this point, the diagnosis of FIP can be made with reasonable certainty.5
The diagnosis of feline infectious peritonitis is typically based on the gross lesions and histopathology in combination with molecular diagnostics and/or immunohohistochemistry.5 Other ancillary tests such as serology (e.g. ELISA) or fluorescent antibody testing can help support the diagnosis of FIP,5 but may be less reliable.
Contributing Institution: The University of Georgia, College of Veterinary Medicine, Department of Pathology, Tifton Veterinary Diagnostic & Investigational Laboratory, Tifton, GA 31793; http://www.vet.uga.edu/dlab/tifton/index.php
JPC Diagnosis: Lung: Phlebitis, necrotizing and
lymphohistiocytic, diffuse, severe, with fibrinoid necrosis and marked alveolar
and interstitial edema.
JPC Comment: The
contributor has provided a fine overview of the disease of feline infectious
peritonitis. Over the last decade, extensive research has been provided
insight into the ?internal mutation theory? that the mutations transforming
FeCV to FIPV occur internally within each individual. Three different genes
have been identified have been identified with this conversion.3
The ORF 3c gene (who protein product is unknown) was the first mutated gene to
be identified, and approximately 2/3 of FIP viruses have ORF3c truncating
mutations resulted in the ability to replicate in macrophages. (Other mutated
ORF 3c genes which are not truncated (in this case causing a premature stop
codons) do not confer this ability on the virus.3
Multiple mutations of the S gene, which encodes the fusion protein, has been
identified in mutated forms in FIPVs and in FIP-infected cats, but not in
FECVs. Single nucleotide alterations, including at the S1/S2 cleavage have
been associated with macrophage tropism. 3
Macrophage activation is one of the most important drivers of FIP in the cat. The typical vasculitis in FIP is that as seen in this case, a vasculitis almost exclusively affecting veins and driven by virus-infected macrophages.1 This phlebitis has been purported to develop as a result of interaction between the viral-infected monocytes and activated endothelial cells. The preponderance of vascular lesion is limited to veins in a number of organs (kidney, lung, meninges, eyes, liver) suggests that not all endothelial cells share responsiveness to macrophages-secreted cytokines.1 The incredible outpouring of fluid from affected vessels in the wet form of FIP (and likely the tremendous amount of fluid in the lungs of FIP cats such as this submission) has been associated with overproduction of vascular endothelial growth factor by virally-infected macrophages. Other cytokines overproduced by macrophages in FIP-infected cats include TNF-alpha, GM-CSF and G-CSF.1 Macrophages secrete the adhesion molecule CD18 in order to attached to activated endothelium, and matrix malloproteinases to digested the vascular basement membrane at sites of emigration. The relative lack of lymphocytes in these lesions helps distinguish this lesion from a true immune-medicated vasculitis.1
References:
1. Kipar A, Meli ML. Feline infectious peritonitis: still an enigma? Vet Pathol 2014, 51(2):505-526.
2. Pearks Wilkerson AJ, Teeling EC, Troyer JL, et al. Coronavirus outbreak in cheetahs: Lessons for SARS. Current Biolo. 2004; 14, R227?R228.
3. Pedersen NC. An update on feline infectious peritonitis: Virology and Immunopathogenesis. The Vet J. 201 (2014) 123?132.
4. Pedersen NC. An update on feline infectious peritonitis: Diagnostics and Therapeutics. The Vet J. 201 (2014) 133?141.
5. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. 6th ed. Elsevier: St. Louis, MO; 2016; 2: 253-255.
6. Zachary JF. Mechanisms of microbial infections. In: Zachary, JF ed. Pathologic Basis of Veterinary Disease. 6thed. Elsevier: St. Louis, MO; 2017; 217-218.