Signalment:
Gross Description:
- Severe, diffuse splenomegaly
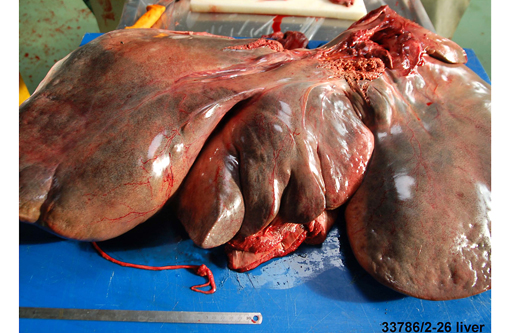
- Severe, diffuse hepatomegaly; liver appeared yellow to pink in color with rounded margins
- Multifocal white, 2 cm to 4 cm, well demarcated, 1 mm thick plaque-like lesions on pulmonary and diaphragmatic pleura
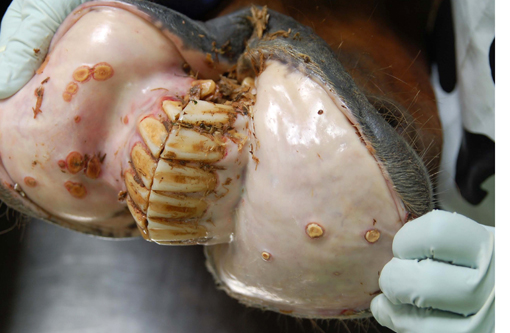
- Numerous irregularly round, yellow to grey, 0.5 to 1.5 cm, plaque-like lesions on the mucosa of the lip, oral cavity, esophagus, small intestine and large intestine
- Severe distention of large intestine with liquid contents
- Plaque-like lesions were also evident on respiratory mucosa of the nasal cavity, larynx, trachea and bronchi
- Marked (up to 5 L) serosanguinous effusion in the abdomen
- Marked (up to 500 mL) serosanguinous effusion in the thorax
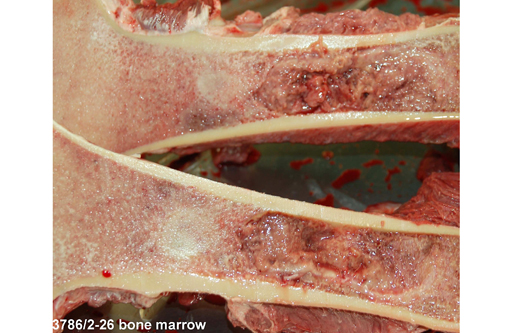
- Replacement of femoral bone marrow by a proliferative lesion
Histopathologic Description:
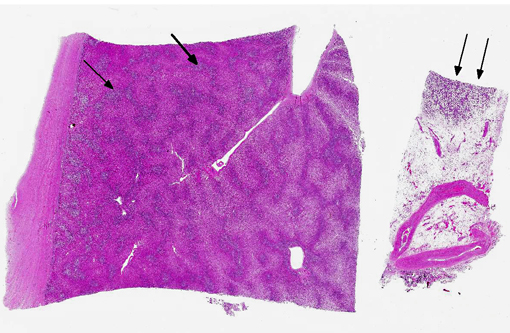
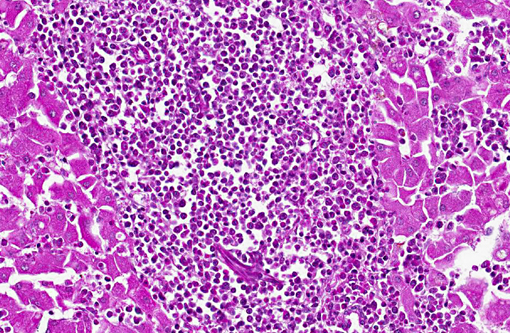
Bone marrow: Bone marrow is effaced by the same neoplastic population described in the liver and there is an absence of erythroid precursors and megakaryocytes (myelophthisis). Blast cells compose up to 80% of neoplastic cells.
Spleen (tissue not submitted): A similar neoplastic population markedly expands the red pulp, filling the sinuses, expanding the splenic cords and multifocally replacing splenic trabeculae. There is diffuse atrophy of the white pulp.Â
Small intestine (tissue not submitted): The neoplastic cells multifocally infiltrate the mucosa and submucosa, forming flattened nodular lesions. The mucosal epithelium is severely and diffusely necrotic.
Lung (tissue not submitted): Neoplastic cells diffusely expand the alveolar walls and are present within the vessels.
Cytology: Imprint with blood coagulum show numerous irregularly round, 20 to 25 μm neoplastic cells, characterized by moderate amount of blue cytoplasm and a round, often indented 15 to 20 μm nucleus, with clumped chromatin and an occasionally distinct nucleolus.
Morphologic Diagnosis:
Lab Results:
Special stains:
- Giemsa: no cytoplasmic granules were evident in neoplastic cells.
- Toluidine blue: no cytoplasmic metachromatic granules were evident in neoplastic cells
Immunohistochemistry:
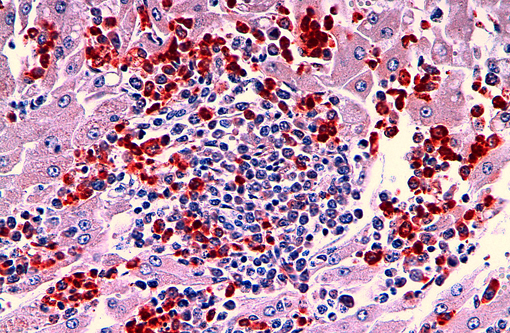
- Myeloperoxidase (M3/38 antibody clone from Cedarlanes): at least 20% of neoplastic cells in the liver and bone marrow were strongly positive.
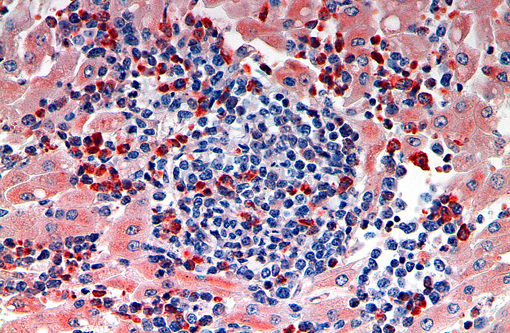
- Lysozyme: at least 40% of neoplastic cells in liver and bone marrow were positive.
- CD79 and CD5: neoplastic cells in liver and bone marrow were negative. Occasional CD79 positive lymphocytes were associated with the neoplastic population.
Condition:
Contributor Comment:
The most common lymphoproliferative disorders in horses are lymphoid leukemia, plasma cell or multiple myeloma and lymphoma.(3) Lymphoma is the most common hematopoietic neoplasia in horses and usually involves lymphoid organs, without leukemia, although bone marrow may be affected after metastasis.(3)
The following outline summarizes the classification scheme of acute myeloid leukemia according to World Health Organization (WHO) criteria:
AML M0: Acute myeloid leukemia/undifferentiated leukemia
- Greater than or equal to 90% of myeloid cells are blasts
- Less than or equal to 5% of blasts in circulating blood stain with myeloperoxidase
- No Auer rods are seen
AML M1: Acute myeloid leukemia without maturation
- There is a predominance of blasts in circulating blood and bone marrow with less than 10% having cytoplasmic granulation
- At least 5% of the malignant blast population stains with Sudan Black and myeloperoxidase
- Reported in young mature dogs and cats, with increased frequency in males
- Swine are occasionally affected; rarely other domestic animal species
- In humans, at least 3% of bone marrow blasts label with myeloperoxidase
- Auer rods may be present
AML M2: Acute myeloid leukemia with maturation
- Approximately 3080% of the myeloid cells are blasts, with at least 10% of neoplastic cells showing maturation (promyelocytes or beyond)
- At least 50% stain with myeloperoxidase
- May have Auer rods
AML M3: Acute promyelocytic leukemia
- There is a predominance of promyelocytes in both in the circulating blood and bone marrow
- There is strong cytochemical staining for myeloperoxidase
- Rare disease of young mature animals
- Commonly recognized in dogs, cats and swine
AML M4: Acute myelomonocytic leukemia
- Both granulocytic and monocytoid differentiation occurs
- Rare disease recognized in dogs, cat, horses
- At least 20% of both tumor cell lines stain for the neutrophil series or for the monocytic series
- At least 20% of blast cells in blood or marrow and at least 20% of the bone marrow cells must be of the monocytic lineage to distinguish M2 from M4
AML M5: Acute monocytic leukemia
M5a: poorly differentiated
- At least 20% blast cells in blood or bone marrow
- Poorly differentiated cells (blasts), monoblasts predominate
- In contrast to M4 less than 20% of cells stain with myeloperoxidase
M5b: well differentiated
- At least 20% blast cells in blood or bone marrow
- Promonocytes predominate
- In contrast to M4 less than 20% of cells stain with myeloperoxidase
AML M6: Erythroleukemia
M6a
- More than 50% of the bone marrow is composed of red blood cell precursors
- More than 20% of the non-erythroid cells are myeloblasts
M6b
- Up to 80% of the bone marrow is composed of red blood cell precursors
- Less than 20% of the non-erythroid cells are myeloblasts
AML M7: Megakaryoblastic leukemia
Rare disease, mainly in dogs
- Greater than or equal to 20% blasts in circulating blood or bone marrow and at least 50% of the marrow cells must be of megakaryocytic lineage
- Circulating blasts with abnormal megakaryocytes and fibrosis in the marrow
- Immunoreactivity for CD41, CD42 and CD61
In the present case, no lymphoadenomegaly or splenic nodules were evident at necropsy. Histopathologic evaluation of the lung, spleen, small and large intestine, esophagus, oral mucosa and nasal mucosa (slides not submitted) revealed a similar neoplastic population to the one in the liver and bone marrow.
Impression smears from the intracardiac coagulum demonstrated the presence of numerous neoplastic cells consistent with myeloblasts/monoblasts.
Immunohistochemical staining of the neoplastic cells in liver and bone marrow for CD3 was negative. Occasional lymphocytes admixed with the neoplastic population were positive for CD79. Almost 25% of the neoplastic cells in the liver were positive for myeoloperoxidase and almost 40% were positive for lysozyme.
Based on the immunohistochemical results, a lymphocytic origin of the neoplastic cells can be ruled out. No laboratory tests for the evaluation of alpha napthyl acetate esterase activity or naphthol AS-D-chloroacetate esterase activity were available to assess the proportion of neoplastic cells with monocytic origin. Severe, diffuse infiltration of neoplastic cells in portal areas of the liver is not reported in AML-M2 but is characteristic of AML-M4.
Two different types of acute myeloid leukemia must be considered as differential diagnosis: acute myeloid leukemia with eosinophilic differentiation (AML-M2) and acute myelomonocytic leukemia (AML-M4).
JPC Diagnosis:
Conference Comment:
In addition to the microscopic lesions described by the contributor, some participants noted scattered cytosegrosomes and occasional intracytoplasmic or extracellular blue to purple granular material. Histochemical staining with Von Kossa identified the granular material as mineral; however, we are unsure of the origin or clinical significance of this material.
References:
1. Forbes G, Feary DJ, Savage CJ, Nath L, Church S, Lording P. Acute myeloid leukaemia (M6B: pure acute erythroid leukaemia) in a Thoroughbred foal. Aust Vet J. 2001;89(7):269-272.
2. McManus P. Classification of myeloid neoplasms: a comparative review. Vet Clin Pathol. 2005;34(3):189212.
3. Munox A, Riber C, Trigo P, Castejon F. Hematopoietic neoplasia in horses: myeloproliferative and lymphoproliferative disorders. J Equine Sci. 2009;20(4):59-72.
4. Newlands MC, Cole D. Monocytic leukemia in a horse. Can Vet J. 1995;36:765-766.
5. Valli VEO. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 3. St. Louis, MO: Elsevier Limited; 2007:107-324.