CASE III: 12-143 (JPC 4065944).
Signalment:
2 month old female snowy owl (Bubo scandiacus (formerly Nyctea scandiaca))
History:
This captive bird was one of a clutch of two eggs hatched in the late spring. Owl was vaccinated for WNV 3 weeks prior to death. Found dead in enclosure with no premonitory signs.
Gross Pathology:
At necropsy, there was mildly reduced breast muscle mass with a prominent keel. There was mild hepatosplenomegaly.
Laboratory Results:
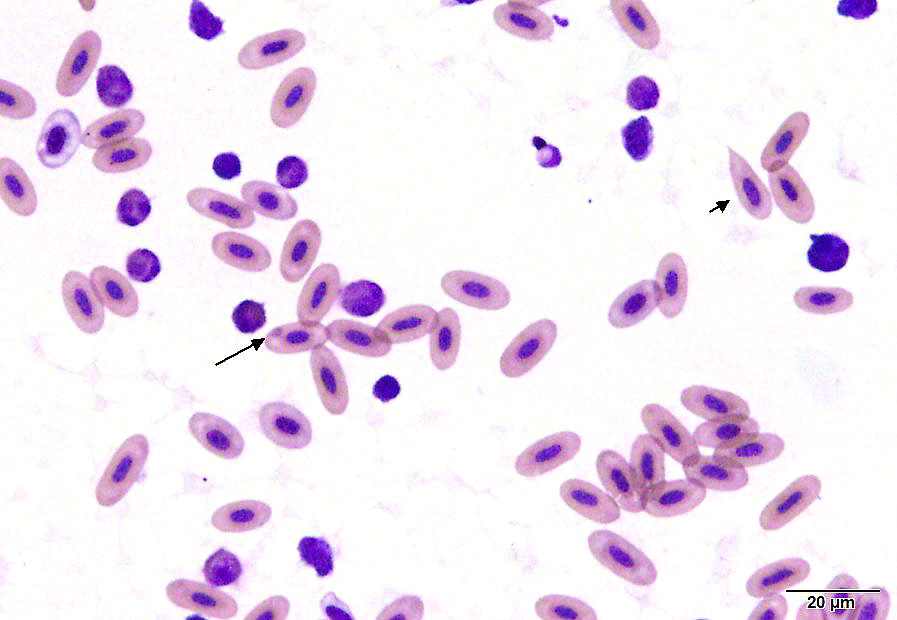
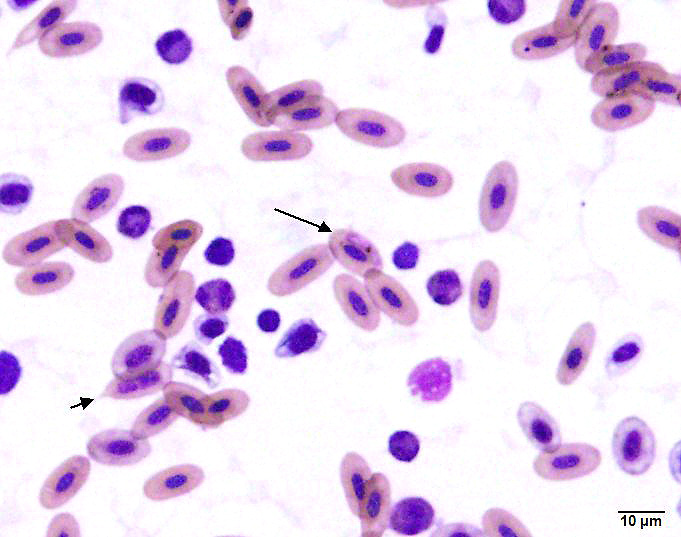
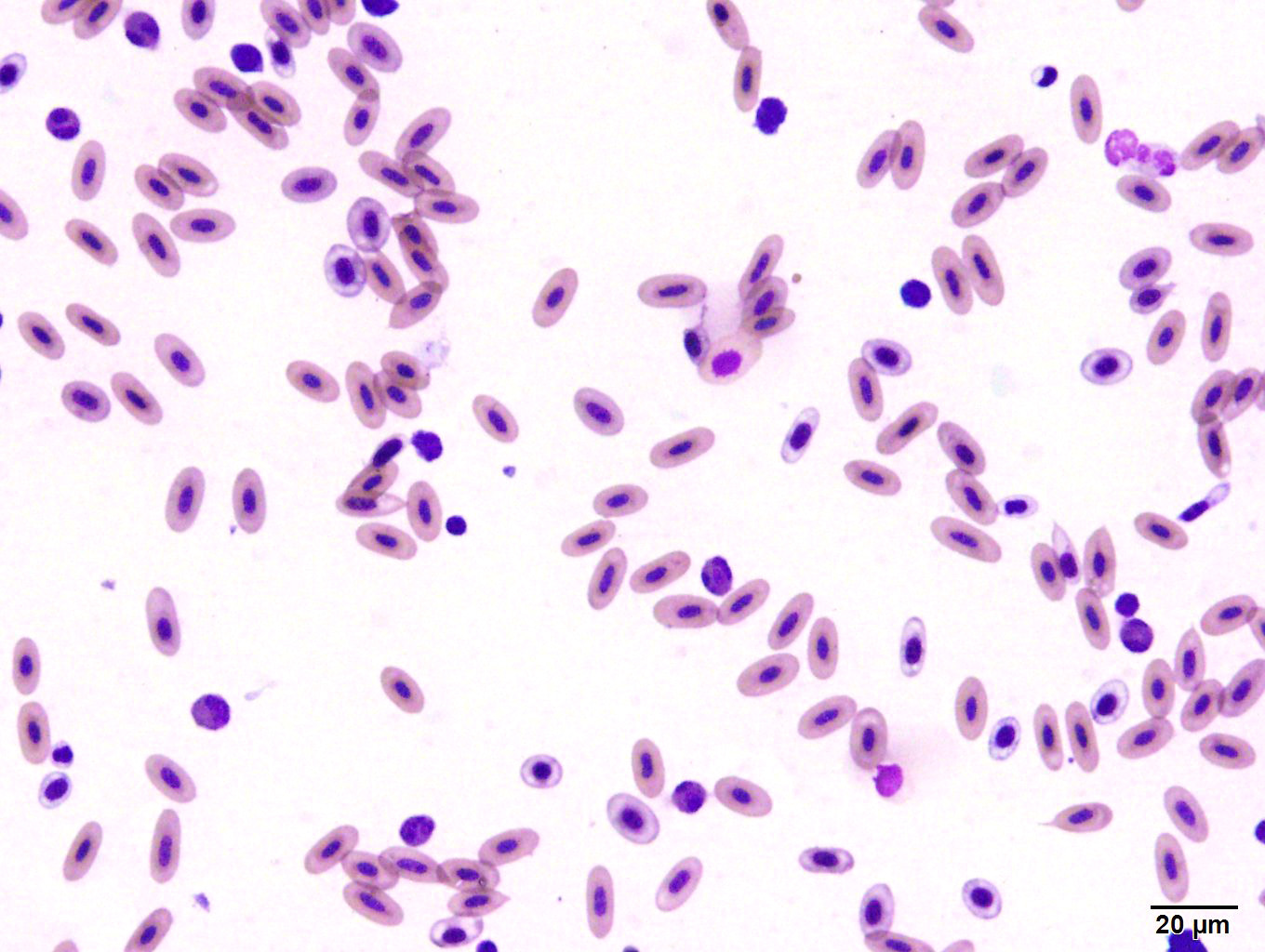
A blood sample obtained from the clutch mate was grossly watery, with a hematocrit
of 5% (normal 38-50%). On a smear there were visible hemoparasites (including trophozoites, round gametocytes and rare schizonts) with hemozoin pigment, as well as polychromasia and schistocytes.
Histopathologic Description:
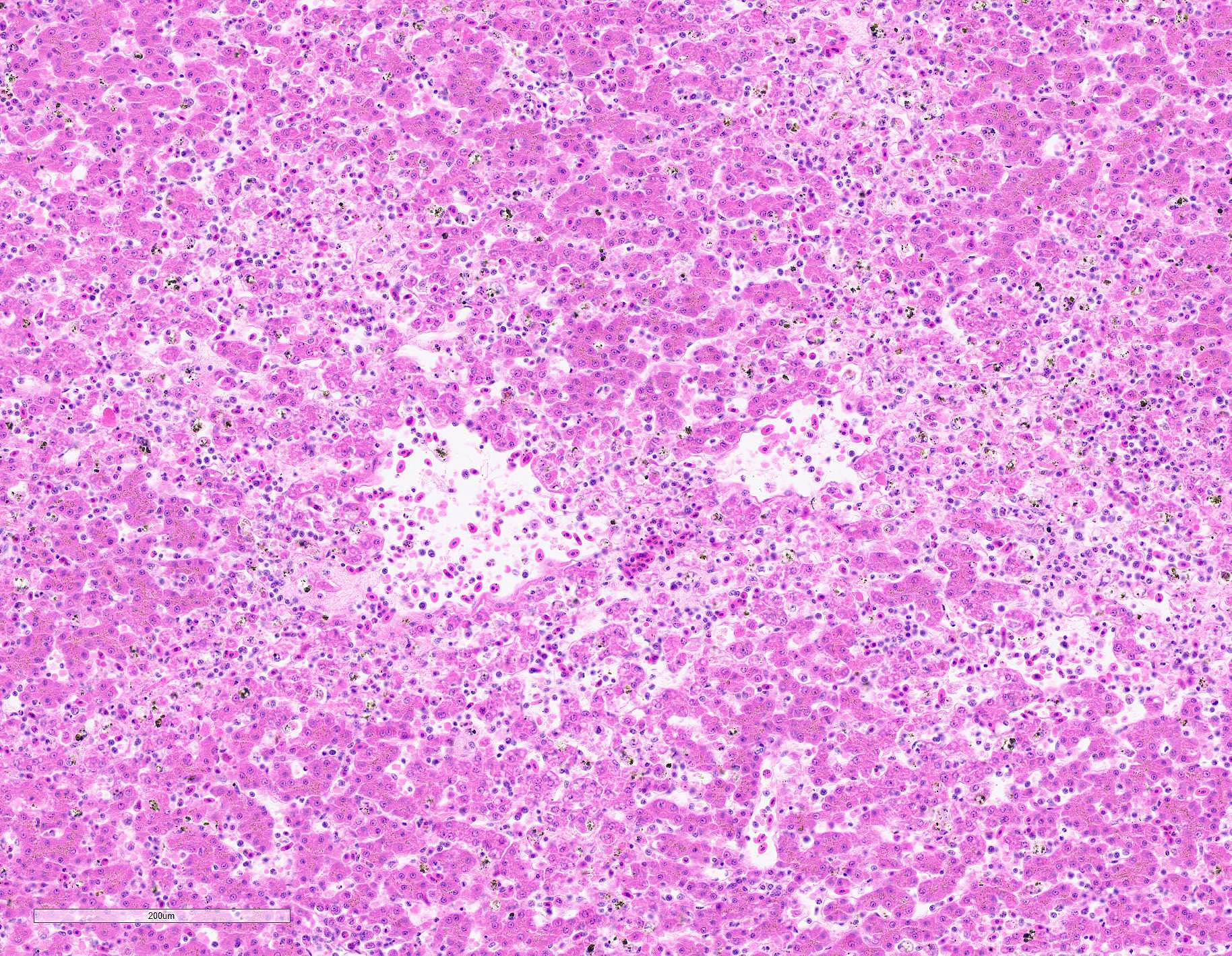
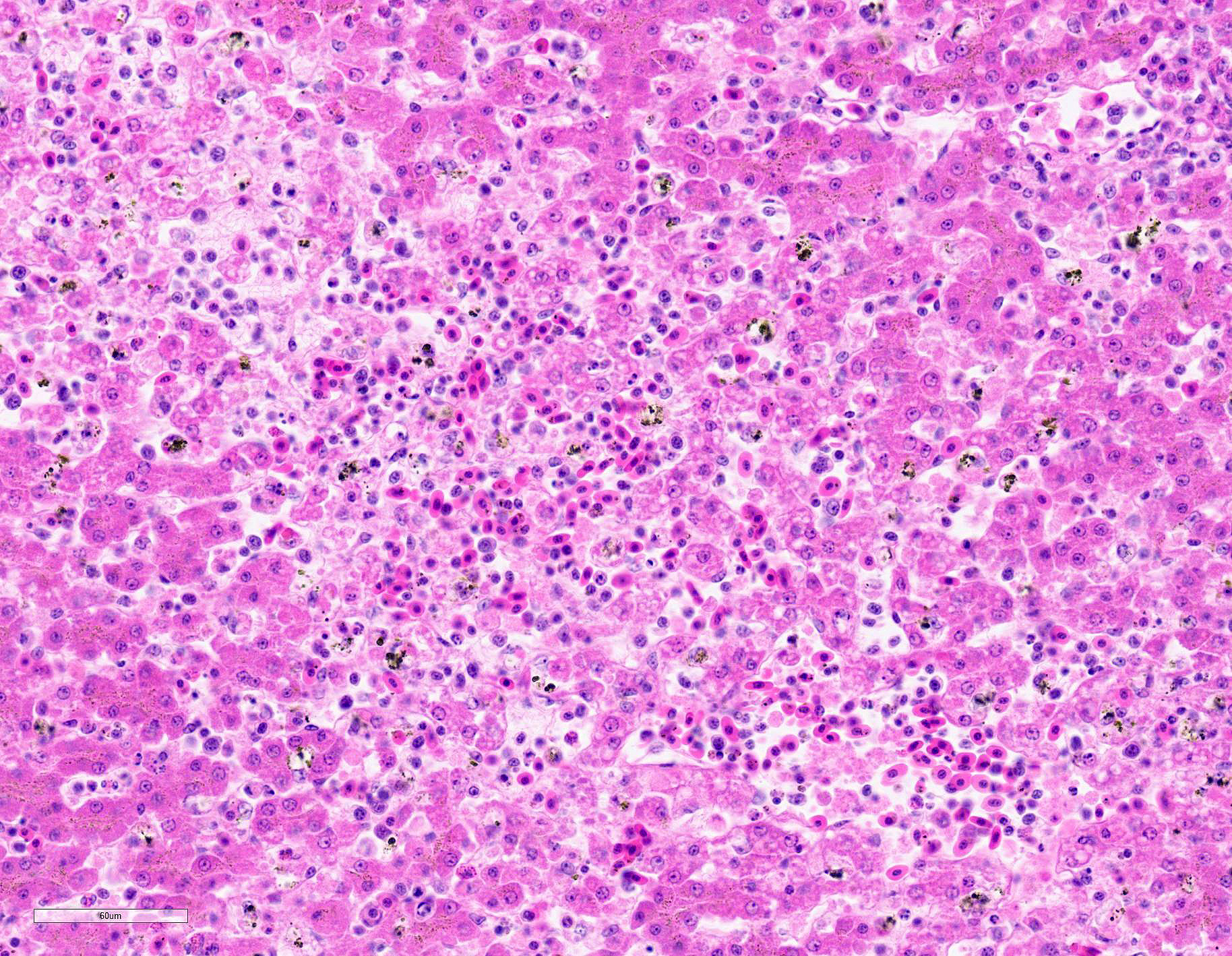
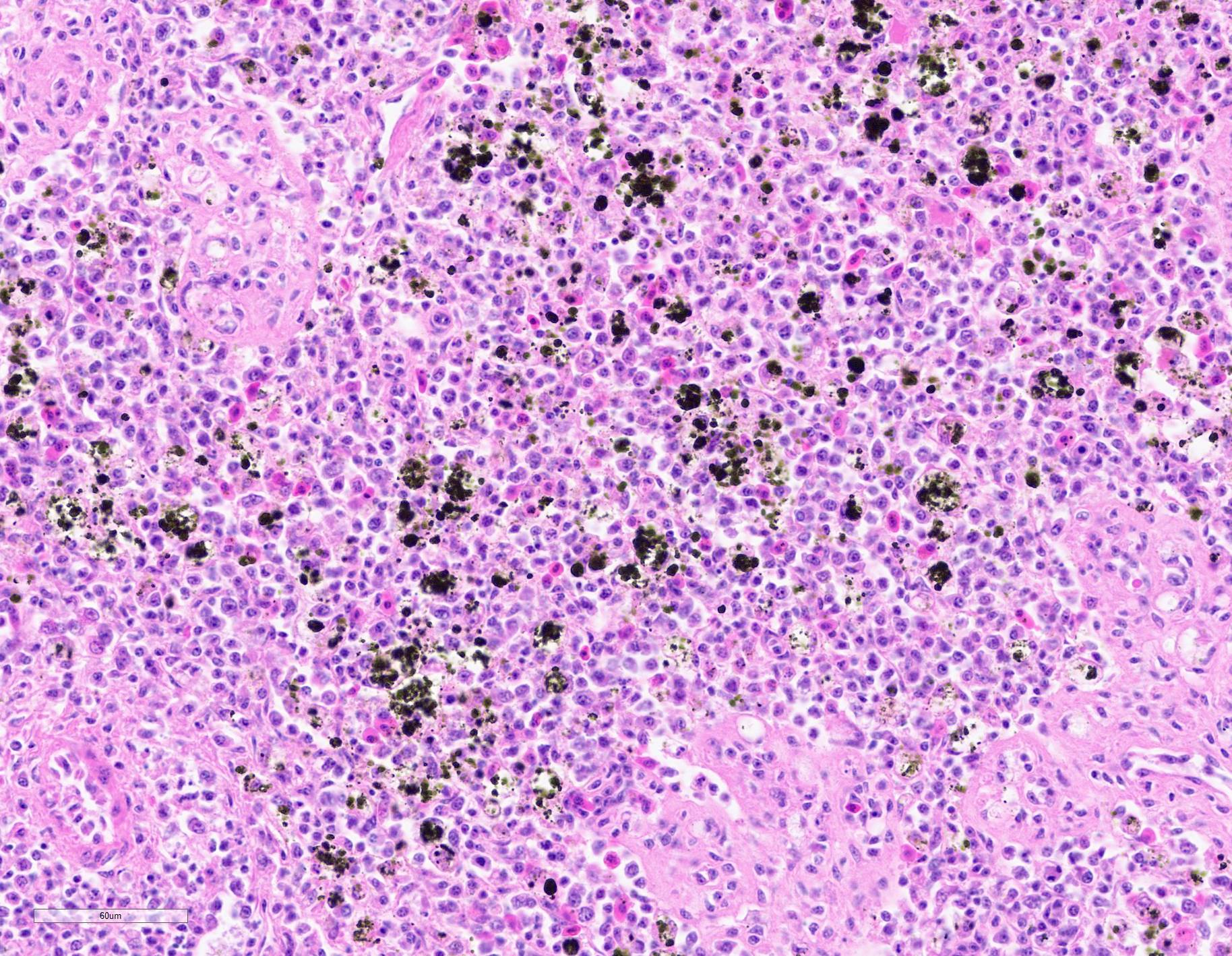
Submitted tissues are liver and spleen. In the liver centrilobular hepatocytes are frequently pale and vacuolated, with dissociation of hepatic cords, rounded swollen hyalinized cells and pyknotic, karyolytic or karyorrhectic nuclei (centrilobular necrosis). Large numbers of Kupffer cells are present in sinusoids. There is abundant refractile finely globular brown-black pigment (hemozoin) with yellow-orange birefringence within Kupffer cells and erythrocytes. Within erythrocytes are frequent protozoal hemoparasites including trophozoites and round gametocytes. Bile casts are visible in some large intrahepatic bile ducts. The splenic red pulp is markedly expanded by large numbers of macrophages, the majority of which are hemozoin-laden. In both organs erythrophagocytosis is evident. Autolysis is present at the margin of the spleen in some sections.
Additional findings included perivascular (ring) hemorrhages in the brain with accumulations of macrophages in the Virchow-Robbins spaces (not submitted).
Contributor?s Morphologic Diagnoses:
Syndrome, hemoparasitism, malaria
Liver and spleen, histiocytosis, diffuse, moderate, with intrahistiocytic hemozoin pigment
Liver, centrilobular hepatocellular degeneration and necrosis, multifocal, acute, mild to moderate
Contributor?s Comment:
The three main erythroparasites of birds are Plasmodium, Leucocytozoon and Haemoproteus.1,2 Evaluation of Giemsa stained blood smears is the standard for diagnosis. Unlike Leucocytozoon and Haemoproteus, Plasmodium undergoes asexual reproduction (merogony) in circulating erythrocytes. Both Plasmodium and Haemoproteus produce hemozoin pigment from the digestion of host cell hemoglobin; Leucocytozoon does not. Haemoproteus forms large sausage shaped gametocytes that partially or completely envelop the erythrocyte nucleus, generally without displacing it; hemozoin pigment granules only appear late within development.
Plasmodium are generally host adapted, causing subclinical infections. Epizootics occur when the parasite and/or mosquito vector are introduced to new areas (such as the Hawaiian Islands), or when hosts native to areas that lack the parasite or requisite mosquito vectors are moved (classically penguins displayed in temperate zoos). After infective sporozoites of Plasmodium are injected by the mosquito, the sporozoites invade local fibroblasts and macrophages and undergo asexual reproduction (merogony), releasing merozoites that invade macrophages for second generation merogony and dissemination.2 Second generation merozoites invade capillary endothelial cells or hepatocytes in the liver (exoerythrocytic merogony). Released merozoites may re-invade endothelium or may invade erythrocytes for asexual merogony or sexual gametogenesis.
Leucocytozoon form large megalomeronts (megaloschizonts) in infected tissues, while Haemoproteus forms sinuous schizonts in lung capillary endothelium (both absent in this case). All three parasites have been reported in captive snowy owls.4,5 The young age in this bird was likely a contributing factor. All three blood parasites have also been identified in other species of owls.6,9 Hepatosplenomegaly with pigmentation is the classic gross appearance of malaria.
Centrilobular hepatocellular degeneration and necrosis in the liver is consistent with ischemia secondary to severe anemia. The centrilobular hepatocytes are located the furthest from the arterial blood supply, and consequently have the lowest oxygen delivery within the lobule.3 Paradoxically, these hepatocytes also have the largest concentration of phase I cytochrome p450s.
Contributing Institution:
Department of Comparative Medicine
Penn State College of Medicine
Penn State Hershey Medical Center
http://www.hmc.psu.edu/comparativemedicine/
JPC Diagnosis:
1. Liver, spleen, macrophages and erythro-cytes: Hemozoin pigment.
2. Liver: Necrosis, multifocal to coalescing, with hemorrhage and extramedullary hematopoiesis.
3. Spleen: Extramedullary hematopoiesis, multifocal, moderate, with erythrophago-cytosis.
JPC Comment:
The contributor provides an excellent summary of three main erythroparasites of birds: Plasmodium, Leucocytozoon and Haemoproteus.
Avian malaria was discovered in 1885, shortly following Alfonse Laveran's discovery of protozoans as the causative agent of human malaria. As a result, avians were subsequently utilized as the primary animal model for malaria research until being overtaken by rodents following the discovery of Plasmodium parasites in Central African rats in 1948. By 1940, the genera responsible for avian malaria were separated into those utilized today: Plasmodium, Haemoproteus, and Leukocytozoon.8
In regard to Plasmodium, the parasite has been identified in over 800 species of birds and on every continent, with the exception of Antarctica where the mosquito vectors responsible for its transmission do not occur. As noted by the contributor, species native to Antarctica relocated to temperate regions (such as penguins) are also susceptible.8
|
As with avians, malaria continues to be a scourge of the human population with over 40% of the world?s population at risk. In 2019, there were 229 million cases and 409,000 deaths attributed to the disease, with children most commonly affected. Dozens of species within genus Plasmodium have been identified within avian species while five are known to be pathogenic in humans: P. falciparum, vivax, ovale, malariae, and occasionally knowlesi. Amongst these five species, P. falciparum is the most prevalent and is often fatal.7 |
Ronald Ross, an army surgeon working in India in 1898, utilized avian models to conclusively prove malaria?s transmission by mosquito vectors. Frustrated by a lack of willing human participants, Ross turned to avian models and later stated, "I should have been wise to have begun my researches in birds in 1895." His research demonstrated mosquitoes were required to obtain blood meals from separate hosts to complete the cycle of transmission. Following an initial blood meal from an infected host, male and female gametocytes ingested by the mosquito differentiate into gametes within the midgut and fuse, forming a zygote. Zygotes subsequently develop into motile ookinetes and migrate to the stomach wall where they form oocytes. After several days, each oocyte produces thousands of sporozoites that eventually localize to the mosquito's salivary glands, ready to infect the next susceptible vertebrate host from which the mosquito takes a second blood meal. Plasmodium sp. then undergo the lifecycle previously described by the contributor within the vertebrate host, facilitating subsequent transmission to additional mosquitoes and completing the cycle of transmission. Prior to this discovery, humans were believed become infected by drinking water contaminated by dead infected mosquitoes.8
Due to the disruption of quinine supplies from Indonesia during World War I, avians were utilized as the first animal models for the development of synthetic antimalarial medications such as plasmoquin and atabrin. In addition, early research in regard to development of antimalarial resistance was conducted during the 1920s and 1930s by administering gradually increasing doses of the medications to infected canerys.8
A hallmark feature of malaria is the presence of black pigment (hemozoin) in tissue sections, which was first noted by Meckel in 1847 in post-mortem blood and splenic samples. Virchow attributed this pigment to malaria two years later. The pigment was believed to be hematin (ferriprotoporphyrin IX hydroxide) until Carbone identified spectroscopic differences between hematin and hemozoin in 1891. Ronald Ross, whose aforementioned efforts proved the transmission cycle between mosquitoes and vertebrates, identified the pigment within the mosquitoes in 1897, further supporting the insect?s role as a vector.7
Hemozoin plays a significant role in the survival of Plasmodium sp. The organism digests up to 75% of the hemoglobin within erythrocytes, resulting in the release of free heme (FeII-protoporphyrin IX) which left unsequestered induces the formation of free radicals as the result of free iron and the Fenton reaction. However, the parasite neutralizes this threat by dimerizing heme to form Fe III-protoporphyrin, which is then stored in insoluable sub-micron sized hemozoin crystals in parasitic digestive vacuoles. Interestingly, quinolines and other antimalarials inhibit the formation of hemozoin within the digestive vacuole, which eventually perforates with spillage of toxic substances such as iron into the parasite's cytoplasm.7
During the conference, participants discussed the significance of hemozoin
pigment in tissue sections such as in this case. Although the presence of
hemozoin significantly increases the index of suspicion of avian malaria, the
pigment is not a pathognomonic lesion. As noted by the moderator, the parasite
is often difficult to identify in tissue sections and advanced diagnostics,
such as PCR, are often required for definitive diagnosis.
There was spirited discussion amongst participants in regard to the pattern of distribution of hepatic necrosis in this case, with multifocal to coalescing being preferred over centrilobular by a majority participants. However, the pathogenesis associated with this pattern of distribution is unclear given the most likely underlying cause of hepatic necrosis in this case is hypoxia secondary to anemia, which is typically associated with centrilobular necrosis. Although this pattern may have manifested as the result of a comorbidity, a battery of histochemical stains including Gram, acid fast, Giemsa, periodic acid-Schiff, and rhodanine did not reveal additional significant findings in examined sections.
References:
1. Hemosporidiosis. In: Friend M, Franson JC, eds. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. Madison, WI: US Geological Survey, Biological Resources Division; 1999: 193-200.
2. Atkinson CT, Thomas NJ, Hunter DB: Parasitic Diseases of Wild Birds. Ames, IA: Wiley-Blackwell; 2008.
3. Cullen JM: Liver, Biliary System, and Exocrine Pancreas. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease, fourth edition. fourth ed. St. Louis, MO: Mosby Elsevier; 2007: 393-462.
4. Evans M, Otter A: Fatal combined infection with Haemoproteus noctuae and Leucocytozoon ziemanni in juvenile snowy owls (Nyctea scandiaca). Vet Rec 1998:143(3):72-76.
5. Harasym CA: West Nile virus and hemoparasites in captive snowy owls (Bubo scandiacus)--management strategies to optimize survival. Can Vet J 2008:49(11):1136-1138.
6. Ishak HD, Dumbacher JP, Anderson NL, Keane JJ, Valkiunas G, Haig SM, et al.: Blood parasites in owls with conservation implications for the Spotted Owl (Strix occidentalis). PLoS One 2008:3(5):e2304.
7. Kapishnikov S, Hempelmann E, Elbaum M, Als-Nielsen J, Leiserowitz L. Malaria Pigment Crystals: The Achilles' Heel of the Malaria Parasite. ChemMedChem. 2021;16(10):1515-1532.
8. Rivero A, Gandon S. Evolutionary Ecology of Avian Malaria: Past to Present. Trends Parasitol. 2018;34(8):712-726.
9. Tavernier P, Saggese M, Van Wettere A, Redig P: Malaria in an eastern screech owl (Otus asio). Avian Dis 2005:49(3):433-435.