Signalment:
The owners noted depression and dysuria. Following three days of progressive signs to include lethargy and decreased thirst, the referring veterinarian examined the animal. A fever of 103.3 °F was recorded, along with a decreased appetite, trouble prehending hay and grain, and teeth grinding. The veterinarian treated the animal with sulfa antibiotics and flunixin meglumine (Banamine-�-�). The next day the horse was more lethargic, reluctant to move, and had a temperature of 101.5°F.
On day 4, the horse was referred to the University of Tennessee (UT) Large Animal Clinic. Upon presentation, the horse was depressed and lethargic, had a weak gait, and dragged both toes of the pelvic limbs at the walk. The neurologic exam revealed a grade III-IV weakness of all four limbs with variable ataxia. The tongue tone was weak, especially to the right, and the horse had difficulty prehending food. There were intermittent fasciculations of the facial muscles, but no other cranial nerve deficits were noted. Additionally, the mucous membranes were icteric and injected, and the horse had multiple abrasions on the tongue and lips and hemorrhages on the gums. Rectal examination, abdominocentesis, equine infectious anemia titers, and upper airway endoscopy were within normal limits. Gastroscopy revealed small ulcers along the lesser curvature of the stomach. Treatments at the UT College of Veterinary Medicine included DMSO, flunixin meglumine (Banamine-�-�), trimethoprim sulfa, IV fluids, dexamethasone, penicillin, and gentamicin (Gentocin-�-�). Despite therapies, the horse became more ataxic and weak, to the point of falling down. He was maintained in the sling overnight, and by the morning of the 6th day, he was head pressing and completely unaware of his surroundings.
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Rabies is a zoonosis with one of the highest fatality rates.(3) The rabies virus belongs to the genus Lyssavirus of the Rhabdoviridae family and is classically spread by a bite from an infected animal.(10) The virus replicates locally before moving along peripheral nerves, where it binds to the nicotinic acetylcholine receptors at the neuromuscular junctions. The virus moves via peripheral nerves toward the central nervous system (CNS) by retrograde axoplasmic transport.(10) Once in the spinal cord, movement occurs using both anterograde and retrograde axoplasmic flow. The virus can also move along peripheral nerves so that the salivary glands become involved, allowing transmission to occur through saliva.(10) This early spread of virus allows for dissemination of infection often before severe clinical signs and immune responses develop.
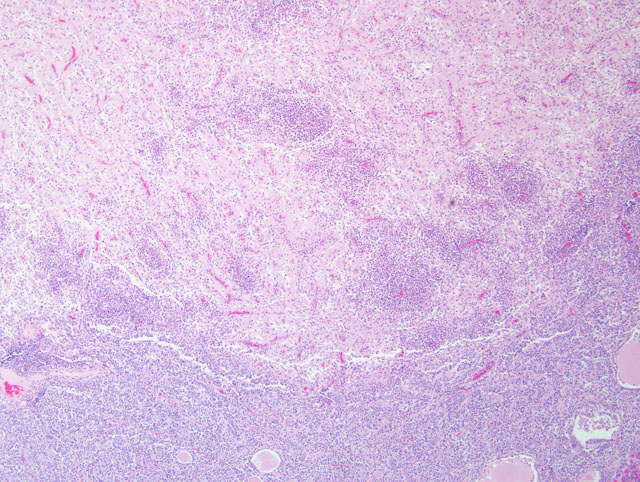
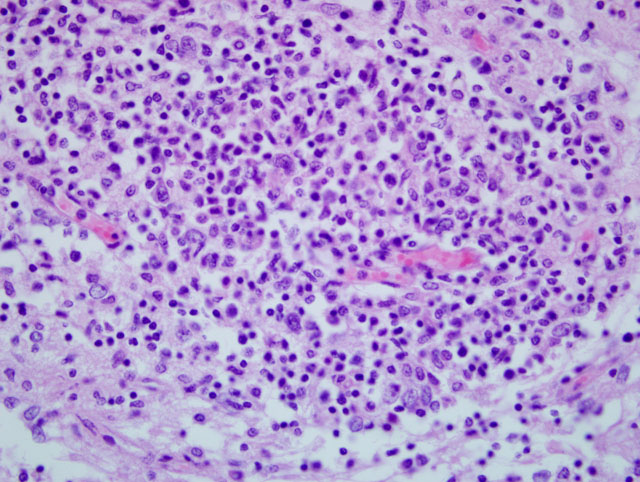
There are typically no gross lesions in rabies cases. Microscopic lesions are variable and include non suppurative leptomeningitis with perivascular cuffs, neuronal degeneration and neuronophagia, gliosis, malacia of the spinal cord grey matter, and ganglioneuritis. Infected neurons may contain intracytoplasmic acidophilic inclusions (Negri bodies), which, while pathognomonic for rabies, are not present in all cases. Negri bodies tend to occur in large neurons, particularly in the pyramidal neurons of the hippocampus, neurons of the medulla oblongata and Purkinje cells of the cerebellum. Hence, these locations are preferred for microscopic or florescent antibody diagnosis of rabies.
Negri bodies were identified in the brain of 10/21 horses examined in one large study.(3) It was thought that they occurred more commonly in horses that survived longer than 4 days.(3) Negri body size may be affected by length of clinical disease and stage of infection when the animal was euthanized.(2) Detection of Negri bodies with hematoxylin and eosin and Sellers stain is limited, as it detected only 50-80% of positive samples, gave false positive results, and had reduced effectiveness in autolyzed samples.(1)
Dr. Adelchi Negri identified the intracytoplasmic inclusions in infected neurons that bear his name in 1903. Even today, the true significance of these structures is still mysterious. It seemed to Negri that fixed strains (passaged in the laboratory) of rabies were less likely to produce Negri bodies because neurons were destroyed early and that the street strains (natural disease) favored their formation, partly because they caused less significant neuronal damage.(6) Rabies in horses in particular has been associated with a spectrum of clinical signs that include, but are not limited to, ataxia, recumbency, pharyngeal paralysis, fever, hyperesthesia, loss of tail and/or anal sphincter tone, progressive paresis, muscle tremors, sweating, anorexia, colic, and lameness.3 Paresis and hind limb ataxia are the most commonly observed clinical signs.(3) In equine cases, where a CSF tap is performed, pleocytosis with lymphocyte prominence and fewer mononuclear cells, macrophages, and neutrophils is documented. The clinical signs may be related to the concentration of inoculated virus, the pathogenicity of the strain, and the proximity of CNS tissue to the site of inoculation. The spinal cord form and dumb form are much more common than the furious form in horses.(3)
Differentials for CNS disease in horses include vertebral malformation, trauma, infections, abiotrophy, and degenerative or idiopathic lesions. Viruses to consider include rabies, EEE, WEE, St. Louis encephalitis, Louisiana virus, snowshoe hare virus, Cache Valley virus, and Main Drain virus.(5) In one large study, wobbler syndrome and equine protozoal myelitis were the most common diagnoses.(5)
The nicotinic acetylcholine receptors have been proposed as the rabies virus receptors.(3) The virus may influence secretion of neuro-modulators and thereby induce functional impairments at sites remote from the site of viral replication. Toll-like receptor 3 has also been implicated in the spatial arrangement of rabies virus-induced Negri bodies and overall success of viral replication.(9) Viruses can exploit cellular proteins for their own benefit.(9)
Immunoperoxidase has been determined to be a useful, accurate, and rapid method for rabies diagnosis.(1) Immunoperoxidase is thought to be more sensitive in early diagnoses of suspected cases in which conventional histology and IFA might not detect viral antigens. In a small case series of four IFA-confirmed rabies cases, IMHC on paraffin tissue detected all rabies cases. Only 2/4 cases had Negri bodies.(5) Similarly, immunoperoxidase on 40 rabies cases showed a specificity of 100% and a sensitivity of 97.6%. Additionally, in another case series, 39/40 cases were positive for rabies with immunoperoxidase, making it a reliable diagnostic technique.(4) Negri bodies were seen in only a fraction of these cases (10/17).(4) Viral antigens were detected in dendrites, axons, glial cells, granular cells in Ammons horn, pyramidal cells and pericaryons of neurons in stratum gangliosum and stratum granulosum of cerebellar cortex.(1)
The amount of rabies antigen varies depending on location within the brain. Following testing of 252 confirmed rabies cases, the thalamus, pons, and medulla were the most reliable parts of brain for testing. Previously, the hippocampus was recommended due to the need to find large inclusion bodies, which occurred at the highest frequency at this site.(2) Current recommendations suggest that the hippocampus and brainstem be sampled.(2)
JPC Diagnosis:
Conference Comment:
Several participants included rabies virus infection in their differential diagnosis list along with other causes of viral encephalitis in the horse. There are two biotypes of rabies virus: fixed virus and street virus. As mentioned by the contributor, the fixed virus is stable and used for developing vaccine strains. The street virus is the wild-type virus involved in disease outbreaks.
Upon inoculation of a susceptible animal, the virus initially replicates in myocytes before budding from the muscle cell and infecting nerves at the neuromuscular junction.(10) The rabies virus glycoprotein receptors for neuronal cell adhesion molecule (NCAM) and the p75 neurotrophin receptor convey the neurotropism displayed by the virus.8 Retrograde axonal transport may involve virus phosphoprotein interaction with microtubule motor protein dynein LC8. Once the virus reaches the CNS, transmission of the virus between neurons results in ascending and descending spread of the virus and precipitating the typical clinical signs and pathologic changes.(10)
The exact mechanism by which rabies causes neuronal lesions and ultimately death is unknown. One possibility is marked viral-induced down-regulation of genes in the brain; affected genes are often involved in regulating cellular metabolism, growth and differentiation. Elevation in the nitric oxide content in rabies-infected brains suggests nitric oxide neurotoxicity. Mouse models of rabies infection demonstrate virus-induced apoptosis, another possible mechanism of neuronal injury and lesions.(10)
References:
2. Bingham J, van der Merwe M. Distribution of rabies antigen in infected brain material: determining the reliability of different regions of the brain for rabies fluorescent antibody test. J Virol Methods. 2002;101:85-94.
3. Green SL, Smith LL, Vernau W, Beacock SM. Rabies of horses: 21 cases (1970-1990). J Am Vet Med Assoc. 1992;200:1133-1137.
4. Hamir AN, Moser G. Immunoperoxidase test for rabies: utility as a diagnostic test. J Vet Diagn Invest. 1994;6:148-152.
5. Hamir AN, Moser G, Rupprecht CE. A five year (1985-1989) retrospective study of equine neurological disease with special reference to rabies. J Comp Pathol. 1992;106:411-421.
6. Kristensson K, Dastur DK, Manghani DK, Tsiang H, Bentivoglio M. Rabies: interactions between neurons and viruses: a review of the history of Negri inclusion bodies. Neuropathol Appl Neurobiol. 1996;22:179-187.
7. Kulonen K, Fekadu M, Whitfield S, Warner CK. An evaluation of immunofluorescence and PCR methods for detection of rabies in archival Carnoy-fixed paraffin embedded brain tissues. Zentralbl Veterinarmed B. 1999;46:151-155.
8. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 1. Philadelphia, PA: Elsevier Ltd; 2007:413-416.
9. Menager P, Roux P, Megret F, et al. Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog. 2009;5:e1000315 Epub ahead of print 2009 Feb 27.
10.In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:833-971.