Signalment:
Seven-year-old quarter horse mare (
Equus ferus caballus).In June 2015,
the horse presented to the referring veterinarian with bilateral conjunctivitis
that progressed to severe anterior uveitis in the left eye. Foot abscesses,
distal limb cellulitis, mandibular lymphadenopathy, nasal discharge, and hives
developed subsequently. Treatments
included ceftiofur, oxytetracycline,
dexa-methasone, nonsteroidal anti-inflammatory drugs, and a two-week course of
doxycycline. Despite treatment, the horse remained hyperfibrinogenemic at
800-1300 mg/dL and developed narcolepsy a few months later. Due to health
concerns and the poor prognosis, the horse was euthanized in January 2016 and
submitted to Cornell Animal Health Diagnostic Center for necropsy and tissue
collection.
Gross Description:
There
was approximately 200 mL of yellow tinged transparent fluid (serous effusion)
within the peritoneal cavity. The capsular surface of the liver was diffusely
thickened, mottled white to tan to purple to black. There were thousands of
multifocal to coalescing, generalized, white, 1-3 mm, hard white nodules along
the capsular surface with a few dozen similar nodules within the parenchyma.
Similar nodules were present in the thymus and surrounding the mediastinal fat
and in all lung lobes. These nodules were presumed to be parasitic granulomas,
which were confirmed histologically. Evidence of chronic laminitis was present
in both forelimbs. The brain was grossly normal.
Histopathologic Description:
Brain,
cerebrum, and cerebellum: Diffusely, meninges of the cerebellum and cerebrum
are markedly expanded by a dense infiltrate of lymphocytes, macrophages, fewer
neutrophils, and rare plasma cells interspersed with rare wispy spirochetal
bacteria. Within the neuroparenchyma, the Virchow-Robin spaces of blood vessels
are surrounded by a similar inflammatory infiltrate. Blood vessels are often
prominent, characterized by endothelial hypertrophy and have variable
branching. In the white and grey matters are increased numbers of enlarged
glial cells with increased eosinophilic cytoplasm (astrocytes, presumptive).
Multifocally within the choroid plexus are clusters of lymphocytes and
histiocytes, along with few eosinophils.
Morphologic Diagnosis:
1. Brain, cerebrum, and cerebellum: Severe,
multifocal to coalescing, chronic lymphohistiocytic neutrophilic meningo-encephalitis
with lymphohistiocytic eosinophilic choroid plexitis, branching blood vessels,
astrocytosis, and rare intralesional spirochetal organisms. Other final
morphologic diagnoses (slides not included):
2. Lymphohistoicytic
meningo-myeloencephalitis and radiculoneuritis of the spinal cord and ganglia,
respectively
3. Moderate,
multifocal, chronic lymphocytic hypophysitis
4. Multifocal,
chronic parasitic granulomas in the liver, lung, and thymus
5. Chronic lymphoplasmacytic
portal hepatitis with capsular and bridging fibrosis
6. Multifocal
laminitis and chronic foot abscess
7. Lymphoid
depletion in spleen and thymus
Lab Results:
Bloodwork
in early December 2015 revealed elevated gamma glutamyl transferase (GGT) at 61
U/L (normal range 9-24 U/L), rising globulins at 2.3 g/dL from a previous value
of 1.6 g/dL (normal range 2.8-4.7 g/dL), and a lymphocyte count of 1,940
cell/uL, up from a previous count of 1,380 cells/uL (normal range 1,000-4,900 cells/uL;
lymphopenia is <1,500 cells/uL).
B cell
concentration was markedly decreased at 19 cells/uL of 1,940 total
lymphocytes/uL (0.98% B cells), with 1.0% CD19 B cells (median, CI = 9.0%,
2.0%), 0.2% CD21 B cells (median, CI = 10.2%, 4.2%), and 0.9% IgM B cells
(median, CI = 10.2%, 2.1%).
The CD4+ and
CD8+ T-cell distributions were slightly increased, and the CD4/CD8 ratio was
within the normal reference interval.
Serum IgG
concentration was markedly decreased at 423 mg/dL (median, CI = 1,760 mg/dL, 603
mg/dL) and serum IgM concentration was within the normal reference interval at
63 mg/dL (median, CI = 100 mg/dL, 50 mg/dL; deficiency is < 25 mg/dL).
Bacterial
cultures and virus isolation of brain tissue were both negative. A quantitative
PCR for Borrelia burgdorferi yielded a CT value of 32 (positive result).
Condition:
Meningoencephalitis/Mutated feline enteric coronavirus (FIP)
Contributor Comment:
The histochemical
stain of a section of brain with Modified Steiner silver stain and
immunohistochemical (IHC) stain for Borrelia burgdorferi confirmed
spiral organisms within areas of inflammation in the meninges of the cerebellum
and cerebrum. IHC stains of section of cerebrum for eastern equine encephalitis
virus, equine herpes virus-1, West Nile virus, and rabies virus yielded no
immunoreactivity. Inflammatory cells were strongly immuno-reactive to CD3 and
IBA1; however, rare CD20 and no Pax5 immunoreactivity were detected, confirming
lack of plasma cells in areas of inflammation and consistent with common
variable immunodeficiency (CVID).
CVID is a
primary immunodeficiency disease of humans and horses that encompass a group of
heterogenous disorders characterized by hypo-gammaglobulinemia. Generally, at
least two isotypes of antibodies are affected, although IgG deficiency alone is
recognized. Human CVID patients often present with recurrent respiratory
infections and have a high frequency of autoimmune and lympho-proliferative
disease.1,8,9 It is one of the most common primary immuno-deficiencies
reported in humans, with an incidence rate of 1 in 25,000 humans. In horses,
CVID is a rare condition with relatively few cases reported,1,2,3,10,14
though the Equine Immunology Laboratory at Cornell College of Veterinary
Medicine has diagnosed this condition in over 50 horses since 2002 and has been
actively investigating potential genetic and epigenetic mechanisms of disease.12
Current research on equine CVID focuses on the disruption of B cell development
in the bone marrow, and has identified decreased mRNA expression and incomplete
demethylation of the PAX5 gene, required for commitment and
differentiation of B cells.12,13
Like human
patients, horses clinically manifest with recurrent infections of the
respiratory tract. In addition, persistent bacterial meningitis has been
associated with infection by common skin contaminants such as Staphylococcus
spp.,3,10 while Borrelia burgdorferi has been highly
suspected in other cases of meningitis. One case report of CNS and PNS
inflammation in a CVID horse documented a positive Western blot analysis result
with low to moderate Borrelia burgdorferi antibody response in serum and
a positive PCR assay result from CSF using primers for the outer surface
protein A (ospA) gene.5 These tests confirm exposure to the
bacterium; however, neither test demonstrates active Borrelia burgdorferi
infection within areas of CNS inflammation. Lyme neuroborreliosis
in horses, as with most species, is characterized by suppurative or
non-suppurative, lymphoplasmacytic, histiocytic perivascular to diffuse
inflammation most severely affecting the CNS, including the meninges, ganglia,
and cranial and spinal nerve roots, with varying degrees of necrosis, fibrosis,
and neuro-parenchymal invasion.4
In
the present case, the inflammation is predominately lymphocytic and histiocytic
and the distribution includes the spinal cord and ganglia, meninges, choroid
plexus, pituitary gland, and neuroparenchyma. By histochemistry and
immunohistochemistry, rare spirochetal organisms were present within areas of
perivascular inflammation, while a quantitative PCR confirmed the presence of Borrelia
burgdorferi nucleic acid in the affected cerebrum . The history of
uveitis and narcolepsy, the clinical data, histologic
findings of severe meningo-myeloencephalitis, choroid plexitis, and
hypophysitis, and ancillary testing are consistent with Lyme neuroborreliosis.4,11
The severe inflammation, fibrosis and parasitic granulomas in
the liver, lung, and thymus are attributed to massive parasitic migration; a
finding consistent with CVID and a lack of antibody response to parasitic
antigens.2,14 The lack of humoral immunity, the primary host defense
mechanism against Borrelia burgdorferi, likely contributed to chronic
Lyme disease in this horse with CVID.
JPC Diagnosis:
Cerebrum: Chorio-meningoencephalitis, lymphohistiocytic,
multifocal to coalescing, marked, quarter horse, Equus ferus caballus.
Conference Comment:
Lyme neuro-borreliosis is an uncommon manifestation of Lyme
disease caused by Borrelia burgdorferi sensu lato infection in
the nervous system, and is typically associated with immunosuppression in
horses, humans, and experimental laboratory animal models.4-6 The
contributor provides an outstanding demonstration of that patho-genesis in this
case of natural infection in a horse with common variable immuno-deficiency
(CVID). As mentioned above, CVID is associated with a late-onset B cell
lymphopenia and hypo-gammaglobulinemia with marked decrease in serum IgG. CVID
typically manifests as opportunistic recurrent pneumonia, septicemia, and
meningitis.14
a
White-footed
mice are the principal reservoir host for B. burgdorferi in the endemic
Northeastern United States, and the bacteria are transferred to susceptible
host species by the Ixodes sp. tick vector. B. burgdorferi localizes in the digestive tract of ixodid ticks via
its outer surface protein A (OspA)
after feeding on an infected reservoir host.7 When the vector
attaches to a susceptible mammalian host and takes a blood meal, there is a
subsequent increase in temperature within the tick digestive tract.
This change in temperature represses OspA expression and induces OspC
synthesis.
This new conformation allows the spirochete to
localize to the salivary glands of the tick. Interestingly, this change in
conformation can take as long as 48 hours to complete, necessitating the
prolonged attachment of the tick to the host. The spirochete then enters the
host via the ticks salivary secretions during feeding.7
Previous
reports of borreliosis in horses have documented arthritis, uveitis,
encephalitis, and ataxia.5 Uveitis, present in this case, is the
most common reported extra-neural manifestation of B. burgdorferi
infection in horses, but is rarely reported in other species.6 The most common mani-festation of disease in dogs is
polyarthritis, with fewer cases of membranoproliferative glomerulonephritis.4-6
Equine neuro-borreliosis is challenging to diagnose clinically due to the wide
variability in clinical presentation and current lack of reliable antemortem
diagnostic tests; however, the conference moderator instructed that the index
of suspicion for Lyme disease should be high in horses that present with
neurologic deficits and concurrent uveitis.4-6
Few conference participants included Lyme disease as a
differential diagnosis in this case. Most favored a viral encephalitis caused
by an alphavirus (EEE, WEE, VEE), rabies, or West Nile virus due to the
relatively non-specific lymphohistiocytic inflammation in this case. Others
included equine protozoal myelitis caused by Sarcocystis neurona;
however, one would expect to see necrotizing granulomatous and eosinophilic
lesions, which are not a feature of this case.6 In conjunction with
the excellent images provided by the contributor, the Joint Pathology Center
ran a Warthin-Starry silver stain, which highlights numerous argyrophilic
spirochetes consistent with B. burgdorferi within the inflamed
neuroparenchyma. This case demonstrates the importance of including Lyme
disease as a differential diagnosis in horses with neurologic disease.
References:
1. Ardeniz
O, Cunningham-Rundles C. Granulomatous disease in common variable
immunodeficiency. Clin Immunol. 2009; 133:198-201.
2. Flaminio
MJBF, LaCombe V, Kohn CW, et al. Common variable immuno-deficiency in a horse. J
Am Vet Med Assoc. 2002; 9:1296-1302.
3. Flaminio
MJBF, Tallbridge RL, Salles-Gomes COM, et al. Common variable immunodeficiency
in horses is characterized by B cell depletion in primary and secondary
lymphoid tissues. J Clin Immunol. 2009; 9:107-116.
4. Imai
DM, Barr BC, Daft B, et al. Lyme neuroborreliosis in 2 horses. Vet Pathol.
2011; 48:1151-1157.
5. James
FM, Engiles JB, Beech J. Meningitis, cranial neuritis, and radiculoneuritis associated
with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc. 2010;
37:1180-1185.
6. Johnstone
LK, Engiles JB. Retrospective evaluation of horses diagnosed with
neuroborreliosis on postmortem examination: 16 cases (2004-2015). J Vet
Intern Med. 2016; 30:1305-1312.
7. Kurmaran
D, Eswaramoorthy S, et al. Crystal structure of outer surface protein C (OspC)
from the lyme disease spirochete, Borrelia burgdorferi. EMBO J.
2001; 20(5):971-978.
8. Maglione
PJ. Autoimmune and lymphoproliferative complications of common variable
immunodeficiency. Curr Allergy Asthma Rep. 2016; 16:19.
9. Pandit
C, Hsu P, van Asperen P, et al. Respiratory manifestations and management in
children with common variable immunodeficiency. Paediatric Resp Rev.
2016; Epub ahead of print.
10. Pellegrini-Masini
A, Bentz AI, Johns IC, et al. Common variable immuno-deficiency in three horses
with presumptive bacterial meningitis. J Am Vet Med Assoc. 2005;
227:114-122.
11. Priest
HL, Irby NL, Schlafer DH, et al. Diagnosis of Borrelia-associated uveitis
in two horses. Vet Ophthalmol. 2012; 15:398-405.
12. Tallmadge
RL, Shen L, Tseng CT, et al. Bone marrow transcriptome and epigenome profiles
of equine common variable immunodeficiency patients unveil block of B
lymphocyte differentiation. Clin Immunol. 2015; 160:261-276.
13. Tallmadge
RL, Such KA, Miller KC, et al. Expression of essential B cell development genes
in horses with common variable immunodeficiency. Mol Immunol. 2012;
51:169-176.
14. Tennet-Brown
BS, Navas de Solis C, Foreman JH, et al. Common variable immunodeficiency in a
horse with chronic peritonitis. Eq Vet Educ. 2010; 22:383-399.

Click the slide to view.
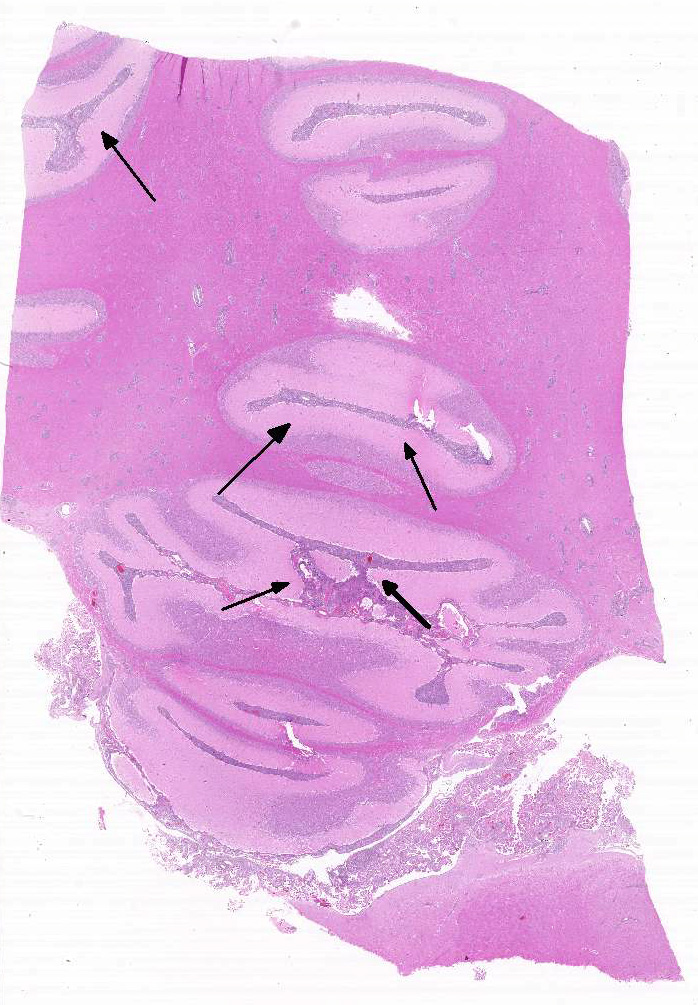
4-1. Cerebellar meninges, horse.

4-2. Cerebellar meninges, horse.

4-3. Cerebellar meninges, horse.
