Signalment:
Gross Description:
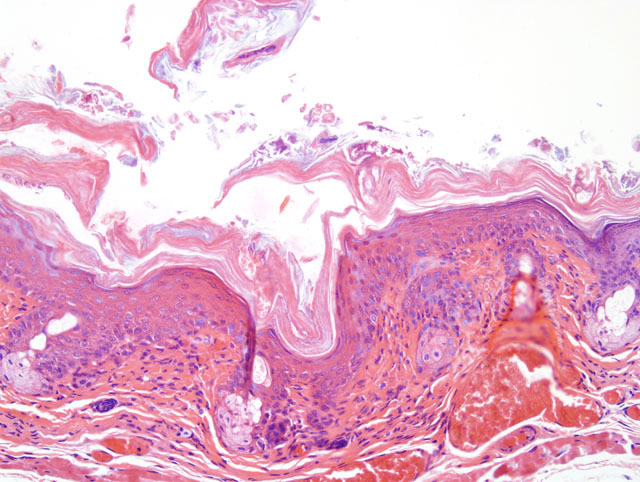
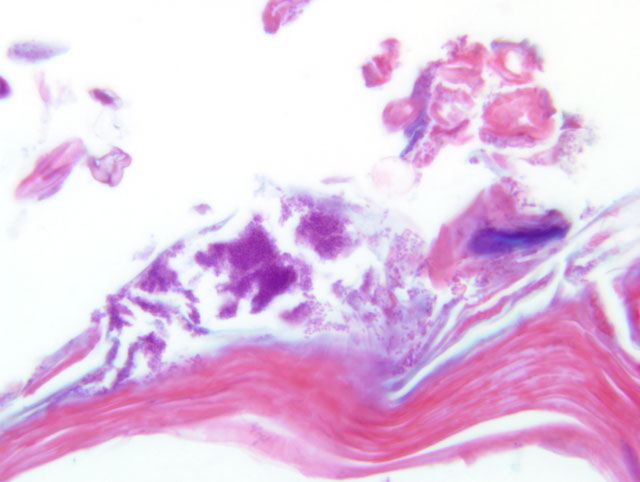
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Brown and Brenn Grams stain: The bacteria are Gram positive. The predominant type is short coryneforms, arranged in typical corynebacterial arrays.
Condition:
Contributor Comment:
Corynebacterial hyperkeratosis has been recognized for decades in athymic nude mice, but also occurs in SCID mice and can be experimentally reproduced in euthymic hairless mice.(1). Morbidity varies, but can be high. Mortality is usually very low except in suckling mice, which can have high mortality. Immunocompetent mice, other than hairless strains, may have asymptomatic infection, but evidence suggests that infection is cleared in these mice.(2,3)
Clinical signs include hyperkeratosis, decreased activity and a wrinkled appearance which probably indicates dehydration. Signs typically appear in susceptible mice about one week after exposure, persist for a week or more, and then usually disappear. Microscopically, the acanthosis remains after the hyperkeratosis resolves; the infection also persists. The mechanism by which C. bovis, a lipophilic bacterium which colonizes the stratum corneum, causes acanthosis and hyperkeratosis is unknown, as is the reason for resolution of the hyperkeratosis.
Histologic features of this case are typical. Hyperkeratosis can be difficult to assess microscopically, is transitory in this disease, and is non-specific; it may be caused by various conditions. Thus, greater diagnostic significance should be given to the acanthosis. Diffuse acanthosis, with a mild non-suppurative dermatitis and the presence of Gram positive coryneform bacteria in the stratum corneum is sufficient for a diagnosis, although in most situations confirmation by culture and or PCR is preferred(4). Other corynebacteria may also colonize the skin surface; our laboratory has identified C. jeikeium, C. minutissimum and Group F2. The latter two organisms are now included in C. amycolatum. None of these are thought to cause skin disease in mice.
In addition to mortality in suckling immunodeficient mice, C. bovis has been reported to slow xenograft growth and increase toxicity observed after chemotherapeutic agents. The mechanism of this is unknown, but the contributor speculates that it might be related to dehydration (symptomatic mice virtually always appear markedly dehydrated, conceivably due to alterations in epidermal barrier function from the diffuse skin disease). Retarded xenograft growth could also possibly be due to non-specific stimulation of host defense mechanisms such as NK cell activity. Non-specific antitumor effects have previously been described for C. kutscheri.(6)
Control of C. bovis infection is difficult. The bacterium is readily transmitted by fomites and is resistant to drying. Our diagnostic laboratory has found positive PCR samples on swabs from cage exteriors, door knobs and even tumor lines passaged as tumor fragments.Â
JPC Diagnosis:
Conference Comment:
Mice and Rats: Corynebacterium kutscheri is a Gram-positive, diphtheroid bacillus that is the cause of pseudotuberculosis in both mice and rats. The normal route of entry of C. kutscheri is through the oral or gastrointestinal mucosa with subsequent hematogenous spread throughout the body. An immunosuppressive event usually precedes clinical disease.(5)
Common gross findings include suppurative bronchopneumonia with randomly distributed caseopurulent nodules; raised, multifocal to coalescing caseopurulent nodules in the heart, liver, or kidney; reactive hyperplasia in lymph nodes near an active site of infection; and pedal arthritis.(5)
On histological section, the bacteria form large colonies surrounded by abundant neutrophils and necrotic debris. Because the disease spreads via sepsis, the suppurative lesions in the lung are randomly distributed. This is an important distinguishing feature between this disease and the disease caused by Mycoplasma pulmonis, which is closely associated with the airways and causes bronchiectasis. The large bacterial colonies of C. kutscheri are pathognomonic and are described as resembling Chinese letters. Interstitial pneumonia is also commonly present in association with C. kutscheri infection and is characterized by hypercellular alveolar septa and pulmonary edema.(5)
Hamsters: These rodents are considered carriers of C. kutscheri but are typically resistant to systemic disease.(5)
References:
2. Gobbi A, Crippa L, Scanziani E: Corynebacterium bovis infection in immunocompetent hirsute mice [see comments]. Lab Anim Sci 1999, 49:209-211
3. Gobbi A, Crippa L, Scanziani E: Corynebacterium bovis infection in waltzing mice [letter; comment]. Lab Anim Sci 1999, 49:132-133
4. Kita E, Nishikawa F, Yagyu Y, Hamuro A, Oku D, Emoto M, Katsui N, Tanikawa I, Kashiba S: Nonspecific stimulation of host defense by Corynebacterium kutscheri. I. Antitumor effect. Nat Immun Cell Growth Regul 1989, 8:313-324
5. Percy DH, Barthold SW: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp.72, 147-148, 192. Blackwell Publishing, Ames, Iowa, 2007
6.Russell, S, Riley, L K, Maddy, A, Clifford, C B, Russell, R J, Franklin, CL, Hook, R R, and Besch-Williford, C L Identification of Corynebacterium bovis as the etiologic agent of hyperkeratosis in nude mice and development of a diagnostic polymerase chain reaction assay. Laboratory Animal Science 48[4], 412. 1998 Ref Type: Abstract